Abstract
We report unusual MRI findings (including those from diffusion-weighted imaging (DWI)) in a patient with recurrent Wernicke's encephalopathy with a remarkable cerebellar lesion. DWI showed high signal intensities in the superior portion of the cerebellar hemisphere and vermis area. After thiamine administration, clinical symptoms improved and the lesions with high signal intensities disappeared on follow-up DWI.
Wernicke's encephalopathy results from a deficiency of thiamine, and presents with three classical signs: progressive unconsciousness, ataxia, and ophthalmoplegia. Lesions are localized to specific parts of the brain, notably the thalamus, periventricular region, and mammillary body.1 It has also been reported that, on autopsy, cerebellar lesions are detected in more than half the patients with the condition. The lesions may be closely related to the presence of ataxia.2 We report DWI findings of a remarkable cerebellar lesion in a patient with recurrent Wernicke's encephalopathy.
A 71-year-old man with a high alcohol consumption was transferred to our hospital due to altered consciousness. He was admitted to the referring medical center twice, in April 2003 and January 2004, because of altered mental state and unsteady gait. At that time, he was diagnosed as having Wernicke's encephalopathy. Before admission to our hospital, he had not eaten meals regularly and had consumed large amounts of alcohol. Two weeks before we saw him, gait disturbance and diplopia had developed. On admission, he was drowsy and disoriented. Because of the gait ataxia, he could not walk without assistance. A neurologic examination showed astasia, paresis of lateral gaze on both sides, nystagmus in the bilateral horizontal direction, decreased deep-tendon reflexes, and hypotonic muscle tone. Bilateral dysfunction was evident when performing rapid alternating movements, the finger-to-nose test, and the heel-to-shin test. Vital signs, electrocardiography, routine laboratory findings, and cerebrospinal fluid analysis were normal. MRI, including DWI, was performed on the first hospital day. Fast fluid attenuated inversion recovery (FLAIR) showed prominent abnormalities in the cerebellum (Fig. 1-A, 1-B, 1-C, 2-A, 2-B, 2-C). The lesions on DWI corresponded to the areas of decreased apparent diffusion coefficient (ADC) values (Fig. 2-D, 2-E, 2-F). There was moderate-to-severe atrophy without significant abnormality on the medial thalamus, periaqueduct, and mammillary body. MR angiography showed normal findings except for stenosis in the distal, left posterior cerebral artery (Fig. 1-G). Infusion of thiamine rapidly improved within 1 day paretic eye movements and nystagmus, and disorientation was diminished after 3 days. Follow-up DWI after 2 weeks of thiamine showed complete recovery of the cerebellar lesion (Fig. 2-G, 2-H, 2-I). Four weeks later, he could walk and limb ataxia had improved. However, abnormal gait due to truncal ataxia and hypotonia persisted.
The patient was considered to have thiamine deficiency due to chronic alcoholism, and clinically presented with acute Wernicke's encephalopathy. In our patient, ophthalmoplegia and confusion rapidly improved after thiamine supplementation, although cerebellar symptoms and Korsakoff's syndrome persisted. MRI intensity changes around the third and fourth ventricles, Sylvian aqueduct, mammillary bodies, medial thalami, and tectum are typical of Wernicke's encephalopathy.8,9 However, in our patient the cerebellar lesion was more prominent than those in the thalamus, periaqueductal area, mamillary body, and tectum. Murata et al. reported a case with remarkable cerebellar lesions on MRI and reported serial MRI/1H-MRS observations.3 Recently several DWI abnormalities in Wernicke's encephalopathy have been reported.4-7 Although similar MRI findings can be seen in Leigh's disease, cytomegalovirus encephalitis, primary cerebral lymphoma, and infarction, we did not consider the MRI findings in our patient to be caused by these other etiologies. First, there was rapid improvement of clinical symptoms after thiamine administration. Second, the cerebellar lesions were not consistent with vascular territory, and there were no abnormalities in the vertebrobasilar arteries. Finally, laboratory and follow-up imaging features were not consistent with infectious, metabolic, or tumorous conditions.
In Wernicke's encephalopathy, the imaging abnormalities parallel pathologic ones.10 Histologically, the earliest findings of Wernicke's encephalopathy are intracellular edema with swelling of astrocytes, oligodendrocytes, myelin sheaths, and neuronal dendrites. More advanced changes are demyelination, petechial hemorrhage, and astrocytic and microglial proliferation with relative neuronal sparing. The exact mechanisms underlying the pathogenesis of the lesions in Wernicke's encephalopathy are incompletely understood.6 Vitamin B1 is a cofactor of the enzyme transketolase and there is decreased activity of the enzyme in the thiamine-deficient state.11 Thiamine-deficient membranes cannot maintain osmotic gradients and this leads to swelling of intracellular and extracellular spaces. In the periventricular regions, there are high rates of thiamine-related glucose metabolism and oxidative metabolism.12 In a thiamine-deficient state this makes these regions more susceptible to transport-mechanism failure. Activity of the thiamine-dependent enzyme alpha-ketoglutarate dehydrogenase, a rate-limiting tricarboxylic acid cycle enzyme, is significantly reduced in autopsied cerebellar vermis samples from patients with Wernicke's encephalopathy.13
Many recent studies have demonstrated the potential of DWI in Wernicke's encephalopathy, in that high signal intensities on DWI and decreased ADC values might indicate the presence of cytotoxic edema.14 However, another report suggests that normal ADC maps and high DWI signals in Wernicke's encephalopathy could result from T2 shine-through effects.4
We do not know why the cerebellar lesion in our patient was more prominent than lesions in the medial thalamus or around the third ventricle. Possibly, chronic neuronal insult may have already degenerated the typical areas involved in this condition so that a cerebellar lesion would then appear relatively prominent.
In conclusion, MRI of patients with Wernicke's encephalopathy may show marked cerebellar abnormalities. Therefore, the possibility of Wernicke's encephalopathy should be considered in patients with these particular MRI findings and presenting typical clinical manifestations.
Figures and Tables
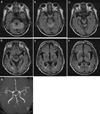 | Figure 1Axial FLAIR images (TR=6500 ms, TE=105 ms) show bilateral symmetrically distributed hyperintensity in the cerebellar hemisphere (A) and the vermis (B, C), and severe atrophy of mammillary body, periaqueduct, and medial thalamus (D, E, F). (G) Bilateral superior cerebellar arteries seem to be patent on MR angiography. |
 | Figure 2DWI images (A, B, C) show bright, high signal intensities in the bilateral cerebellum, while the corresponding apparent diffusion-coefficient values of the lesions (D, E, F) show low signal intensities. Follow-up DWI after 2 weeks of thiamine shows complete recovery of cerebellar lesion (G, H, I). |
References
1. Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke's encephalopathy. N Engl J Med. 1985. 312:1035–1039.
2. Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. 12 Contemporary Neurology Series. 1989. 2nd edn. Philadelphia: FA Davis.
3. Murata T, Fujito T, Kimura H, Omori M, Itoh H, Wada Y. Serial MRI and (1)H-MRS of Wernicke's encephalopathy: report of a case with remarkable cerebellar lesions on MRI. Psychiatry Res. 2001. 108:49–55.

4. Doherty MJ, Watson NF, Uchino K, Hallam DK, Cramer SC. Diffusion abnormalities in patients with Wernicke encephalopathy. Neurology. 2002. 58:655–657.

5. Bergui M, Bradac GB, Zhong JJ, Barbero PA, Durelli L. Diffusion-weighted MR in reversible Wernicke encephalopathy. Neuroradiology. 2001. 43:969–972.

6. Chu K, Kang DW, Kim HJ, Lee YS, Park SH. Diffusionweighted imaging abnormalities in Wernicke encephalopathy: reversible cytotoxic edema? Arch Neurol. 2002. 59:123–127.

7. Niclot P, Guichard JP, Djomby R, Sellier P, Bousser MG, Chabriat H. Transient decrease of water diffusion in Wernicke's encephalopathy. Neuroradiology. 2002. 44:305–307.

8. Watson WD, Verma A, Lenart MJ, Quast TM, Gauerke SJ, McKenna GJ. MRI in acute Wernicke's encephalopathy. Neurology. 2003. 61:527.

9. Opdenakker G, Gelin G, De Surgeloose D, Palmers Y. Wernicke encephalopathy: MR findings in two patients. Eur Radiol. 1999. 9:1620–1624.

10. Suzuki S, Ichijo M, Fujii H, Matsuoka Y, Ogawa Y. Acute Wernicke's encephalopathy: comparison of magnetic resonance images and autopsy findings. Intern Med. 1996. 35:831–834.

11. Dreyfus DM. Thiamine and the nervous system: an overview. J Nutr Sci Vitaminol (Tokyo). 1976. 22:Suppl. 13–16.
12. Witt ED. Neuroanatomical consequences of thiamine deficiency: a comparative analysis. Alcohol Alcohol. 1985. 20:201–221.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download