Abstract
Hyperhomocysteinemia associated with methylene terahydrofolate reductase (MTHFR) mutation can be a risk factor for idiopathic cerebral venous thrombosis. We describe the first case of MTHFR 677TT homozygote with cerebral venous thrombosis and livedo reticularis. A 45-year-old man presented with seizures and mottled-like skin lesions, that were aggravated by cold temperature. Hemorrhagic infarct in the right frontoparietal area with superior sagittal sinus thrombosis was observed. He had hyperhomocysteinemia, low plasma folate level, and MTHFR 677TT homozygote genotype, which might be associated with livedo reticularis and increase the risk for cerebral venous thrombosis.
Hyperhomocysteinemia causes vascular endothelial damage that result in atherosclerosis and ischemic strokes.1 It is also associated with prothrombotic state or venous thromboembolism2 including cerebral venous thrombosis.3 Among the thrombophilic factors with hyperhomocysteinemia, methylene tetrahydrofolate reductase (MTHFR) mutant (C677 → T, homozygote) with low plasma folate concentration increases the risk for cerebral venous thrombosis.4 MTHFR 677TT is thermolabile and sensitive to temperature alteration.5
Livedo reticularis is a skin lesion caused by hyperviscosity in venules.6 It often reflects hypercoagulable states such as cryoglobulinemia or cold agglutininemia.6 However, livedo reticularis with hyperhomocysteinemia or cerebral venous thrombosis is unknown.
We experienced the first case of livedo reticularis and MTHFR 677TT mutant presenting with seizures and venous infarct due to cerebral venous thrombosis.
A 45 year-old man was brought to the emergency room with uncontrolled seizures. Two days ago, sudden paresthesia in left arm developed, which progressed to tonic posturing and leftward head version, followed by a generalized tonic clonic seizure. In the emergency room, he was drowsy but blood pressure was 131/84 mmHg with heart rate of 86, respiratory rate 18, and body temperature 36.6℃. Other physical findings including chest or abdomen revealed no abnormalities. However, mottled bluish discolorations of skin were noted in all extremities, more prominent in both legs and buttock areas (Fig. 1). Neurologic examination showed bilateral gaze-evoked nystagmus. Mild weakness in muscle power was noted in the left extremities, but sensory seemed to be symmetric to painful stimuli. Deep tendon reflexes were hyperactive in both sides. Neck was supple and carotid or ophthalmic bruits were not audible.
T2-weighted MR images showed heterogeneous signals in the right frontal and parietal lobes involving cortical and subcortical regions. Diffusion-weighted images indicated that the lesions were due to both vasogenic and cytotoxic edema. MR venography showed filling defect in superior sagittal sinus (Fig. 2).
The laboratory findings showed hyperhomocysteinemia with plasma homocysteine level of 25.7umol/L (normal range 5~15 umol/L). Low plasma level of folate was also detected (2.2 ng/mL, normal range 3~15 ng/mL). He had MTHFR homozygote mutation (C677 → T) in genetyping analysis. Vitamin B12 level, and other prothrombotic factors such as plasma protein C, S, or antithrombin III levels were normal. Cryoglobulin cold agglutinin, dysfibrinogenemia, activated protein C resistance, prothrombin gene mutation, antineutrophilic cytoplasmic antibody, antinuclear antibody, anti-double strand DNA antibody, lupus anticoagulants, anticardiolipin antibody, or evidence for disseminated intravascular coagulation (DIC) were all negative.
He was treated with intravenous unfractionated heparin and switched to warfarin at the time of discharge. He also received anticonvulsant, carbamazepine 400 mg/day, and supplements of folate, cobalamine and pyridoxine. He was recovered after the treatment without neurological deficit.
We first report a case of cerebral venous thrombosis and livedo reticularis in thrombophilic state with hyperhomocysteinemia and MTHFR 677TT. There was a report with cerebellar infarction and hyperhomocysteinemia having MTHFR 677TT and CBS 1080TT mutant.7 However, cerebral venous thrombosis associated with MTHFR 677TT genotype has not been reported.
There are several reports with cerebral venous thrombosis, related to the thrombophilic state, such as protein C or antithrombin III deficiency or paroxysmal nocturnal hemoglobinuria.9,10,11 However, cerebral venous thrombosis with hyperhomocysteinemia has been reported in one case, in which the patient had homocysteinuria and superior sagittal sinus thrombosis.8
Hyperhomocysteinemia is the newly recognized causes of cerebral venous thrombosis. It represents approximately 30% of the cerebral venous thrombosis.12 In addition to the factor V Leiden and prothrombin gene mutation (PTHRA20210), hyperhomocysteinemia is frequently observed in patients with cerebral venous thrombosis when compared to that of controls (43.3% versus 10%).12 The mechanism of venous thrombosis by hyperhomocysteinemia is unknown. It has been suggested that homocysteine has direct effect on the vascular endothelium and the clotting cascade.13,14 Genetic and nutritional factors are also important in hyperhomocysteinemia.4 Low folate, vitamin B12 or B6 deficiencies are prone to have hyperhomocysteinemia, especially in patient with the MTHFR thermolabile mutant. In our case, the patient had been predisposed to prothrombic state in mild folate deficiency. The mutation of C → T at nucleotide position 677 of the MTHFR gene is responsible for a lower synthesis of methyl-tetrahydrofolate from methylene-tetrahydrofolate.15 Because methyl-tetrahydrofolate is a methyl donor in the methylation reaction of homocysteine to methionine, a decrease of MTHFR activity may result in homocysteine accumulation.15
This patient showed livedo reticularis, which might reflect systemic hypercoagulable states such as thrombocythemia, cryoglobulinemia, cold agglutininemia, lupus erythematosus, anticardiolipin syndrome, disseminated intravascular coagulation, leukemia, or vascular obstructive diseases in arterioles or venules such as thromboemboli, polyarteritis nodosa, rheumatoid vasculitis, livedoid vasculitis, and Sneddon's syndrome.6 In this case, the patient had a history of livedo reticularis prior to the thrombotic event. The supporting evidence of other systemic causes for livedo reticularis were absent on the laboratory tests. We can infer that the livedo reticularis may predict a risk of cerebral venous thrombosis, although whether it directly cause cerebral venous thrombosis remains unknown.
We report the first case of cerebral venous thrombosis associated with MTHFR 677TT mutant in Korea. Livedo reticularis may reflect prothrombotic state caused by hyperhomocysteinemia, which resulted in cerebral venous thrombosis. It is worth screening for the thrombophilic conditions including skin lesion, homocysteine, plasma folate and MTHFR gene mutation for idiopathic cerebral venous thrombosis especially in young patients.
Figures and Tables
 | Figure 1A mottled, net-lke purplish discolorations of both legs suggesting livedo reticularis, which aggravated with cold temperature, but did not disappear after warming-up or elevation of limbs. |
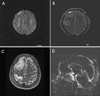 | Figure 2MR images of the patient. Diffusion MRI (A) and ADC map (B) showed vasogenic and cytotoxic edemas on the right frontoparietal lobes which were hemorrhagic infarctions with surrounding edema (C). Brain venography showed filling defects in the superior sagittal sinus, suggesting thrombosis (D). |
References
1. Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke. A meta-analysis. JAMA. 2002. 288:2015–2022.
2. den Heijer M, Koster T, Blom HJ, Bos GM, Briet E, Reitsma PH, et al. Hyperhomocysteinemia as a risk factor for deep vein thrombosis. NEJM. 1996. 334:759–762.
3. Martinelli I, Battagliolo T, Pedotti P, Cattaneo M, Mannucci PM. Hyperhomocysteinemia in cerebral venous thrombosis. Blood. 2003. 102:1363–1366.
4. Cantu C, Alsonso E, Jara A, Martinez L, Rios C, Fernandez Mde L, et al. Hyperhomocsyteinemia, low folate and vitamin B12 concentrations, and methylene tetrahydrofolate reductase mutation in cerebral venous thrombosis. Stroke. 2004. 35:1790–1794.

5. Blom HJ. Mutated 5,10-methylenetetrahydrofolate reductase and moderatehyperhomocysteinemia. Eur J Pediatr. 1998. 157:S131–S134.
6. Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL. Harrison's Principles of Internal Medicine. 2005. 16th ed. United States of America: The McGraw-Hill Companies, Inc;1460–1461.
7. Lee DH, Park CI, Lee IH, Lee JM, Kim SH, Lee JY, et al. An adolescent ischemic stroke patient with hyperhomocysteinemia, MTHFR 677T and CBS 1080TT genotypes. J Korean Neurol Assoc. 2005. 23:382–385.
8. Koo JS, Yoon BW, Kim J. A patient with homocystinuria complicated by superior sagittal sinus thrombosis: a case report. J Korean Neurol Assoc. 1997. 15:1271–1279.
9. Kim SJ, Heo K, Ye JS, Lim SR, Kim OK, Cho HK. A case of deep cerebral venous thrombosis associated with hereditary protein C deficiency. J Korean Neurol Assoc. 1996. 14:567–571.
10. Kim JC, Kang SY, Song HS, Lee SA. Cerebral venous sinus thrombosis associated with antithrombin III deficiency: a case report. J Korean Neurol Assoc. 2000. 18:637–641.
11. Lee MS, In SW, Lee SJ. A case of cerebral venous thrombosis in paroxysmal nocturnal hemoglobinuria. J Korean Neurol Assoc. 1986. 4:246–250.
12. Ventrura P, Covelli M, Marietta M, Panini R, Rosa MC, Salvioli G. Hyperhomocy-steinemia and other newly recognized inherited coagulation disorders (Factor V Leiden and prothrombin gene mutation) in patients with idiopathic cerebral vein thrombosis. Cerebrovasc Dis. 2004. 17:153–159.

13. Cattaneo M. Hyperhomocysteinemia, atherosclerosis and thrombosis. Thromb Haemost. 1999. 81:165–176.

14. Harpel PC, Zhang X, Borth W. Homocysteine and hemostasis: Pathogenic mechanisms predisposing to thrombosis. J Nutr. 1996. 126:1285S–1289S.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download