Abstract
Hemispatial neglect refers to a cognitive disorder in which patients with unilateral brain injury cannot recognize or respond to stimuli located in the contralesional hemispace. Hemispatial neglect in stroke patients is an important predictor for poor functional outcome. Therefore, there is a need for effective treatment for this condition. A number of interventions for hemispatial neglect have been proposed, although an approach resulting in persistent improvement is not available. Of these interventions, our review is focused on caloric stimulation and optokinetic stimulation. These lateralized or direction-specific stimulations of peripheral sensory systems can temporarily improve hemispatial neglect. According to recent functional MRI and PET studies, this improvement might result from the partial (re)activation of a distributed, multisensory vestibular network in the lesioned hemisphere, which is a part of a system that codes ego-centered space. However, much remain unknown regarding exact signal timing and directional selectivity of the network.
Hemispatial neglect refers to a cognitive disorder in which patients with unilateral brain injury fail to report, orient to, or respond to stimuli located in the contralesional hemispace, which is not attributed to primary sensory or motor defects.1 Hemispatial neglect is more severe and frequent after the right than the left hemisphere injury,2 representing one of the major cognitive disorders resulting from right hemisphere damage. Hemispatial neglect usually recovers spontaneously3 but can be persistent in about 10% of thepatients.4
When a horizontal line is presented in front of the patients with hemispatial neglect, they place the bisection mark to the ipsilesional space from the true midpoint (Fig. 1-A). When asked to cancel out lines randomly distributed in an A4 sized paper, they place the marks on the right side of the page (Fig. 1-B).5,6 This rightward bias or preference also occurs when copying figures (Fig. 1-C).7,8 These behaviors seen in test situations may be translated into patients' daily lives. For instance, patients with left hemispatial neglect from a right hemisphere stroke may eat food placed on the right side of the plate. When reading newspapers, they read only the right side of the page. This rightward preference occurs not only for environmental objects but for patient's own body: patients neglect their own left limb, thus fail to shave the left side of their face or do not sleeve their left arm while putting on clothes. Patients with severe left neglect also show anosognosia for hemiplegia in which patients deny their muscle weakness or asomatognosia in which patients deny that their left arm belongs to them. Thus, hemispatial neglect is one of the factors that interfere with rehabilitation,9,10 and serves as one of the poor prognostic factors for stroke patients.11
Considering these clinical implications of hemispatial neglect, it is thus worthwhile developing therapeutic modalities for hemispatial neglect. Since Lawson introduced a method,12 there have been a variety of interventions to ameliorate hemispatial neglect for the last 40 years. While the effectiveness of these interventions is controversial,13 researches are going on worldwide and new methods have been proposed.
We will first describe various methods for improving hemispatial neglect and focus our discussion on the effect of caloric stimulation and optokinetic stimulation (OKS).
The patients with left hemispatial neglect were trained to look at stimuli on the left hemispace.13 Alternatively, cues were placed on the left hemispace such that patients' attention is drawn to the left hemispace. These cue interventions have been proved to be effective in reducing patients' hemispatial neglect. The cues used in those studies included auditory as well as visual ones. The researchers used even motor cues asking to move left arm or leg while performing bisection tasks.14
One of the accounts for hemispatial neglect is the shift of patients' egocentric reference frame to the right. Several methods have been proposed assuming that these may restore the distortion of reference frame. These include caloric stimulation, neck muscle vibration, transcutaneous electric nerve stimulation (TENS), trunk rotation, OKS. Being involved in the generation of information about the position of the head relative to the extrapersonal space, the vestibular system is crucial for the organization of subjective spatial coordinates (egocentric space). Therefore it is likely that the vestibular stimulation produces an attentional bias coherent with the direction of the slow phase of the nystagmus, which may either compensate or increase the attention bias of these patients toward the side ipsilateral to the cerebral lesion.
Recently, repetitive TMS on the left hemisphere (injured left hemisphere) was reported to improve left hemispatial neglect.15
Prism lens can be designed such that objects on the left appear on the right side. Therefore, wearing this prism lens allow patients with left neglect to see objects on the left side. This prism also showed a delayed effect; patients demonstrated improved performances on neglected tasks even after taking the prism off.16
Normally, the superior colliculus plays an important role in mediating orienting behavior to the contralateral space. In an experiment with cats with unilateral cortical lesion (e.g., right cortical lesion) and consequent left-sided neglect, a surgical ablation of left superior colliculus improved the neglect.17 This improvement was explained by a release of inhibition of the right superior colliculus from the left superior colliculus, thus improving leftward orienting mediated by the right superior colliculus. Colliculi receive input mostly from the contralateral eye and left neglect associated with right hemisphere injury would be reduced when the patients' right eye is patched. They observed that right eye patching decreased left neglect, although subsequent studies showed inconsistent results. Instead of using monocular patching, some researchers used hemispatial glasses to block input to the contralateral colliculus18 while others reported that half patch was superior to full patch.19
According to underlying mechanisms of hemispatial neglect, neglect can be divided into perceptual and premotor neglect.20 In perceptual neglect, patients fail to respond to left-sided stimuli since they are not aware of them. On the other hand, in premotor neglect, patients fail to respond to left-sided stimuli since they lack the intent to move despite intact awareness of the target stimuli. It has been hypothesized that dopaminergic circuits play a role in the premotor components of the unilateral neglect syndrome. Indeed, experimental studies demonstrated that monkeys having parkinsonism by infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) via intracarotid artery showed apparent contralesional hemispatial neglect that is probably due to delayed initiation of movements.21 Consistent with these experimentalfindings, unilateral neglect in some human subjects was reduced by administration of dopamine agonist.22,23
The caloric stimulation is ordinarily performed with the subject reclining, head inclined 30 degrees up from horizontal so as to place the horizontal canal in the vertical plane. Water is introduced into the ear canal on one side, either 7℃ above or below the assumed body temperature (30 or 44℃). The flow rate is such that the ear rapidly equilibrates with the water. The water is stopped after 30 seconds, and nystagmus is observed. Nystagmus commonly builds for about 30 seconds, then gradually decays away over roughly 2 minutes.
Vestibular pathways run from the VIIIth nerve and the vestibular nuclei through ascending fibers such as the medial longitudinal fasciculus to the ocular motor nuclei and the supranuclear integration centers in the pontomesencephalic and rostral mesencephalic brainstem. This part represents the three-neuron arc of the vestibulo-ocular reflex, which is embedded in a more complex sensorimotor system responsible for the orientation of eyes, head and body in space with descending input to vestibulospinal projections for head (vestibulocollic reflex) and postural control (vestibulospinal reflexes)24-27 and ascending input to thalamocortical connections for perception.28 Coordination of eye, head and body movements during locomotion is further mediated by corticofugal connections between cortical areas and the vestibular nuclei.27 From the midbrain, ascending fibers reach several multisensory cortical areas through thalamic projections. The two major cortical functions of the vestibular system are the perception of verticality and of self-motion. Perception of verticality relies mainly on otolith input; perception of self-motion involves otolith and semicircular canal input. The multiplicity of representations of vestibular cortex areas has been identified in electrophysiological and tracer studies in animals29-41 and the multisensory neuronal functions of these areas argue for a network of multisensory (vestibular) areas at the cortical level.
A complex network of areas predominantly located in the temporo-insular and temporo-parietal cortex could be delineated in both human hemispheres.42-51 The areas activated during caloric stimulation in humans include the posterior insula (first and second long insular gyri) and the retroinsular regions [representing the parieto-insular-vestibular cortex (PIVC) and the posterior adjacent visual temporal sylvian area36 in the monkey], superior temporal gyrus (STG), parts of the inferior parietal lobule (IPL) representing area 7 in monkeys, intraparietal sulcus representing monkeys' ventral intraparietal area (VIP), post-central and pre-central gyri, anterior insula and adjacent inferior frontal gyrus, anterior cingulate gyrus, precuneus and hippocampus which is most often activated bilaterally (Fig. 3). Simultaneous to these activations, deactivations of areas within the visual and somatosensory systems of both hemispheres were observed.45,52 Activation of the cortical network during vestibular stimulation is not symmetric in both hemispheres. Rather, it depends on three determinants which were defined recently in a study investigating healthy right- and left-handers.49 The determinants were first the subjects' handedness, second the side of the stimulated ear and third the direction of the induced vestibular symptoms. Activation was stronger in the non-dominant hemisphere, in the hemisphere ipsilateral to the stimulated ear, and in the hemisphere ipsilateral to the fast phase of vestibular caloric nystagmus in cases of warm caloric stimulation (Fig. 3).45,49,53
OKS requires a stimulus that fills the field of vision. A common method is for the patient to sit inside a large, patterned optokinetic drum. Virtual reality technology has been used to overcome the cumbersome nature of large mechanical rotating drums. Another method is to rotate the patient at a constant velocity for more than a minute with the eyes open in a lighted room; as the labyrinthine signal dies away, the sustained nystagmus is due to purely visual drives. Small hand-held optokinetic drums or tapes primarily test the pursuit system. The optokinetic response is judged by both the nystagmus during visual stimulation (which in primates consists of pursuit and optokinetic components) and the optokinetic after-nystagmus (OKAN) that occurs after the lights are turned out. It is known that an OKS produces a nystagmus with a slow phase coherent with the movement and a quick phase going back to the initial fixation. This reflex keeps constant the retinal image when the body moves in the external space. It is evoked by continuous retinal signals and not by phasic labyrinthine signals. For this reason it does not exhibit decay after 20-30 s as for the vestibular reflex, but it can be produced for long period of time.
OKS activates directionally-selective retinal ganglion cells that project via the magnocellular layers of the lateral geniculate nucleus to layer 4Cα of striate cortex.54 Some neurons in striate cortex respond to moving visual stimuli, but these cells having small receptive fields, respond only to motion in the frontal plane, and cannot encode higher image velocities. Striate cortex projects both directly and indirectly to the middle temporal visual area (MT or V5)55; in addition, MT receives inputs that bypass striate cortex,56 perhaps via the superior colliculus and pulvinar.57 Neurons in area MT have larger receptive fields than those in striate cortex and encode the speed and direction of target movements in three dimensions.58-60 Experimental lesions in MT corresponding to extrafoveal retina cause a scotoma for motion in the contralateral visual field: stationary objects are perceived appropriately but motion perception is disrupted.61 The consequences of lesions of extrafoveal MT for eye movements are that saccades can still be made accurately to stationary targets in the affected visual field, but moving stimuli cannot be tracked accurately by saccades or smooth pursuit.62 Functional imaging studies have demonstrated the human homologue of area MT which is located at the temporo-parieto-occipital junction, posterior to the superior temporal sulcus, at the junction of Brodmann areas 19, 37 and 39, close to the intersection of the ascending limb of the inferior temporal sulcus and the lateral occipital sulcus.63,64 Patients with cortical lesions have been described who appear to have perceptual65-67 or ocular motor68,69 deficits similar to those reported with MT lesions in monkeys.61,62
Visual area MT, in turn, projects to the medial superior temporal visual area (MST),58,70 which contains neurons that not only encode moving visual stimuli but also appear to carry an eye movement signal.71 Area MST seems to be important for analyzing the optic flow that occurs during locomotion.72,73 Area MST is also important for the generation of smooth pursuit eye movements; lesions here or in the foveal representation of MT cause a deficit primarily of horizontal smooth pursuit for targets moving towards the side of the lesion. In addition, a retinotopic deficit for motion detection, similar to that with extrafoveal lesions of MT, is present for targets presented in the contralateral visual hemifield.62 The human homologue of area MST may lie adjacent to MT.74 Other cortical regions, such as the superior temporal polysensory area,75 visual area 3a, and the superior parietal occipital region76 may also contribute to processing of moving visual stimuli and directing visuospatial attention, but their homologous areas and contributions to human eye movements remain yet to be determined.
MT also projects to the frontal eye field (FEF). MST also receives inputs from its contralateral counterpart. MST projects through the retrolenticular portion of the internal capsule68 and the posterior portion of the cerebral peduncle to the dorsolateral pontine nucleus (DLPN).77-79 The DLPN also receives inputs important for pursuit from the FEF; these inputs descend in the medial portion of the cerebral peduncle. The DLPN projects, mainly contralaterally, to the flocculus, paraflocculus,80 and ventral uvula of the cerebellum; projections also pass to the dorsal vermis.81 The flocculus projects to the ipsilateral vestibular nuclei, which in turn project to the contralateral abducens nucleus. The output of the vestibular nuclei influences the brainstem circuitry that controls eye movements, posture, and perception of self-motion.
Retinal ganglion cells also project to neurons in the nucleus of the optic tract, which in turn project to neurons in the dorsal cap of the inferior olive. Dorsal cap neurons project to Purkinje cells in the contralateral flocculus and nodulus of the cerebellum. Activity of cerebellar Purkinje neurons directly modifies the activity of neurons in the vestibular nuclei. But functional capacity of the subcortical visual pathway in adult humans with normal binocular vision is uncertain.
functional MRI (fMRI) studies using frequency-spoiled single-slice fast low-angle shot (FLASH) pulse sequences and echo planar imaging (EPI) during optokinetic nystagmus (OKN) found bilateral activations in a complex sensorimotor network, especially in the visual cortex, including the motion-sensitive area MT/MST in the occipitotemporal cortex and the adjacent occipitoparietal cortex, as well as ocular motor areas such as supplementary, frontal, and parietal eye fields, and the prefrontal cortex.49,82-84 In the first study, Bucher et al.82 individually compared the signal intensity changes and the extent of activation of each activated area (by counting the number of voxels per cluster in only one slice) in every single subject, and an analysis for repeated measurements revealed no significant difference between rightward or leftward OKN. However, the number of activated voxels on average was smaller in the left hemisphere than in the right hemisphere, and a right hemispheric dominance in the occipitotemporal region was assumed, regardless of the direction of OKN.82 Using a similar type of data analysis in single subjects, the second study confirmed the assumption of a right hemispheric dominance in visual motion-sensitive areas, predominantly in the occipitotemporal cortex and ocular motor and vestibular cortex areas (posterior insula) during horizontal and vertical OKN stimulation.83 In contrast, this hemispheric dominance of cortical ocular motor areas was not found in several fMRI and PET imaging studies that used statistical group analysis techniques to determine the cortical processing of other types of eye movements such as smooth pursuit or voluntary saccades.85-87 Only eye movements in the context of spatial and visual attention tasks showed a right hemispheric dominance.88-90 In one recent study,91 the subtraction analysis between rightward and leftward OKN showed no direction-specific activation of the ocular motor areas such as the frontal, prefrontal, and parietal eye field or other cortical or subcortical areas (Fig. 5-A). Although earlier monkey studies reported that almost all pursuit neurons in the FEF92 and MT/MST had a preferred direction of motion, neurons with different direction preferences obviously lay intermingled within the frontal eye fields and MT/V5 region.59,93 Functional imaging studies have not yet been able to monitor these effects for single neurons, most probably due to insufficient spatial resolution; however, they were able to demonstrate direction-selective imbalance in the area of MT+.94 In conclusion, the absence of a significant difference in the activation pattern of the cortical eye fields supports the view that the processing of eye movements in both horizontal directions is mediated in the same cortical ocular motor areas. Functional scanning studies have often yielded discrepant results, partly reflecting the use of different test paradigms. Another pitfall of functional scanning is that inferred local changes in cerebral metabolism may represent excitation or inhibition. More developed studies with excellent temporal resolution (e.g., magnetoencephalography) may present differential patterns of cerebral activations by directional OKS.
Horizontal OKN led in both directions to a similar nearly symmetrical bilateral pattern of BOLD signal decreases (deactivations) compared to the control condition (Fig 5-B).92 Decreases were located in the posterior part of the corpus callosum and the neighboring lower posterior cingulate gyrus (BA 24/31), partly merging into the hippocampus, and in the optic radiation / tapetum. Additional bilateral deactivations were found in the central sulcus region, predominantly in the postcentral gyri, which could be best attributed to the somatosensory cortex and adjacent parietal areas. Small unilateral signal decreases were located during both OKN directions in the frontal-most and medial part of the right middle frontal gyrus (BA 8). Decreases in the posterior insula region containing the human homolog of the parieto-insular vestibular cortex as described earlier49 were found bilaterally only at a significance level of P≤0.005. Since these activation-deactivation patterns which occurred during visually induced self-motion perception with activations of parietal areas and concurrent deactivations of the multisensory (vestibular) cortex were opposite from those by vestibular stimulation,83,95 a reciprocal inhibitory cortical interaction between the sensory systems was assumed.95
Unilateral vestibular stimulation of the horizontal semicircular canal by caloric irrigation of one ear induces a tonic imbalance in the bilateral vestibular system provoking identical vestibular symptoms as observed with a unilateral vestibular lesion. The direction depends on the water temperature used for caloric irrigation of the horizontal canal (ipsilateral effects with 30℃ cold water; contralateral effects with 40℃ warm water). Unilateral vestibular stimulation in healthy subjects also induces a tonic shift of the average horizontal eye position with the nystagmus.96,97 This lateral bias of eye position is towards the left with left-sided cold caloric stimulation and towards the right with right-sided caloric stimulation. In addition, unilateral caloric stimulation provokes a shift of the exploratory eye movements towards the side of stimulation, leading to asymmetric target search (Fig. 6). A further consequence of unilateral vestibular stimulation in healthy subjects is a tonic bias of spontaneous head orientation around the yaw axis.98 For example, cold caloric stimulation of the right ear provokes a deviation of spontaneous head orientation of 20-30° towards the right.
The ipsilesionally biased field of spontaneous exploration in neglect patients has been demonstrated to be transiently shifted back towards the contralesional side by cold caloric stimulation of the left vestibular organ (Fig. 5).99-105
For the first time, Rubens studied performance on tests of visual neglect and left lateral gaze after caloric stimulation in 18 patients with left-sided visual neglect from strokes.99 Except for one patient with absent vestibulo-ocular responses, all improved during caloric stimulation on the left by cold (LC) or on the right by warm water (RW). During LC and RW caloric stimulation, patients worked from left to right instead of their usual right to left. He interpreted the improvements in spatial exploration as a consequence of the induction of a motor response directed toward the left side. He proposed that caloric stimulation might be of use in training patients with hemispatial neglect to orient toward the affected hemispatial field. Cappa observed improvement of both personal neglect and anosognosia in some neglect patients.100 The latter findings appear inconsistent with any interpretation of this effect as dependent upon ocular nystagmus. In one experiment,105 three patients with a right, predominantly parietal lesion and marked left-sided neglect without visual field defects were asked to direct a laser point to the position which they felt to lie exactly 'straight ahead' of their bodies' orientation. Whereas in both light and darkness, the subjective body orientation was close to the objective body position in the control groups, the three neglect patients localized the body's sagittal midplane approximately 15 degrees to the right of the objective orientation. No relevant differences of 'straight ahead' were found between the neglect patients and controls in the vertical plane. The neglect patients' horizontal displacement of sagittal midplane to the right could be compensated for either by neck muscle vibration or by caloric vestibular stimulation on the left side. When vestibular stimulation was combined with neck muscle vibration, the horizontal deviation linearly combined by adding or neutralizing the effects was observed when both types of stimulation were applied exclusively in the control groups as well as in the neglect patients. Moreover, data analysis revealed that the neglect patients' ipsilesionally displaced subjective body orientation does not result from a disturbed primary perception or disturbed transmission of the vestibular or proprioceptive input from the periphery. The results supported the hypothesis that the essential aspect leading to neglect in brain-damaged patients is a disturbance of those cortical structures that are crucial for transforming the sensory input coordinates from the peripheral sensory organs (the retina, neck muscle spindles and cupulae) into an egocentric, body-centred coordinate frame of reference. In neglect patients the coordinate transformation seems to work with a systematic error and deviation of the spatial reference frame to the ipsilesional side leading to a corresponding displacement of subjective localization of body orientation. It could be concluded further that neck muscle proprioception and vestibular stimulation directly interact in contributing to the subject's mental representation of space. The data suggested that the afferent information from these different input channels is used simultaneously for computing egocentric, body-centered coordinates that allow us to determine our body position in space.
The superior temporal cortex, insula and the temporoparietal junction are not strictly 'vestibular' but rather have a multimodal character representing a significant site for the neural transformation of converging vestibular, auditory, neck proprioceptive and visual input into higher order spatial representations.106 Neurons of these regions provide us with redundant information about the position and motion of our body in space. They seem to play an essential role in adjusting body position relative to external space.
Cold water irrigation on left ear in patients with left hemispatial neglect causes tonic deviation of eyeball to the left and rapid corrective eye movements toward right, presumably shifting the viewer center reference frame to the left resulting in temporary improvement of the signs associated with left neglect.99 Similarly, a leftward moving background or OKS can also induce similar eye movements, i.e., slow leftward and rapid corrective rightward eye movements,13 suggesting that a moving background may have the same beneficial effects on neglect as the caloric stimulation. Several studies have provided support for the postulate that movement of the background can influence the signs associated with hemispatial neglect and can even induce attentional biases in healthy individuals. For example, when patients with neglect were asked to bisect stationary horizontal lines superimposed on an MB, the MB caused their attempts at line bisection to deviate from no movement condition in the direction of MB.13,107,108
According to our literature search, there had been 11 studies that investigated effect of OKS on hemispatial neglect. The first study recruited 33 patients with left neglect who were asked to bisect lines placed on the plexiglass box within which light spots were moving.109 The results showed that leftward moving background improved the left neglect. In this study, all three groups of subjects (normal subjects, patients with neglect and without neglect) were affected by OKS, placing the bisection marks in the same direction of OKS.
Zoccolotti et al110 performed a case study in which visual scanning of a patient with left hemispatial neglect improved by standard training along with OKS which, however, did not affect the patient's anosognosia for visual disturbance.
Two studies done by Vallar et al.111,112 used dependent variable as the pointing task. Ten patients with neglect were asked to point straight ahead with the index finger while watching OKS (moving dots). In the control condition where the dots were stationary, patients pointed to the right. Leftward OKS improved this rightward bias while the rightward OKS aggravated it. These results have clinical implications that OKS can affect even nonsensory components of neglect.
Mattingley et al.107 presented lines on a computer display, with a neutral, static, or slowly drifting, random dot background. The background was moving at speeds that did not elicit optokinetic nystagmus or perceptual aftereffects. Controls were accurate in all conditions, whereas patients with left hemispatial neglect showed a significant leftward shift in bisection errors, when the background was moving leftward. There was no significant effect of rightward motion in comparison with the neutral and static conditions.
Karnath113 asked three patients with neglect to direct a laser point to the position which they felt lay exactly "straight ahead" of their bodies' orientation. Without OKS, the patients' pointing shifted to the right but leftward OKS improved and rightward OKS aggravated this bias.
Bisiach et al.108 superimposed lines printed on a transparent over the OKS generated by computer display. Ten patients with left neglect were asked to bisect lines in this experimental setting. The leftward OKS made the bisection marks shift to the left compared to the neutral condition. However, rather than bisecting accurately, patients bisected to the left of the true midpoint, suggesting that OKS does not correct the distorted representation but that patients' with neglect had the representation that is vulnerable to OKS.
Vallar et al.114 studied two patients with left neglect and reported that leftward OKS improved motor weakness of left hand, albeit transiently. The same group published another article115 that OKS improved left neglect but did not affect position sense (i.e., pointing straight ahead, 30, 60, 90 degree from the midsagittal plane).
Kerkhoff et al.116 requested six patients with left neglect to estimate lengths of lines that were presented on the moving background. In control condition where there was no motion, patients underestimated the lines presented on the left hemispace and overestimated the lines presented on the right hemispace. Leftward moving background restored the misperception of size. In contrast, the rightward moving background aggravated the overestimation of the right-sided lines, which however did not reach significance.
Pizzamiglio et al.117 compared two groups of patients: one group received only conventional spatial scanning while the other group received spatial scanning and OKS. Both groups showed beneficial effects 6 weeks later, but the effects did not differ between the two groups.
Previous studies showed that when the OKS moves leftward or rightward, the line bisection error of neglect patients deviates in the same direction of background movement. Even normal subjects showed the same behavior.117 However, when normal subjects are looking at a stationary object on a moving background, the stationary object appears to move. This perception of illusory motion (IM) might bias the subject's allocation of spatial attention. Our group tested for the first time whether the IM affects line bisection performances in normal subjects.118 Young normal volunteers were asked to bisect stationary lines with a background of horizontal OKS. To maximize IM, the stimuli were generated by computer and displayed on a large screen via a beam projector as illustrated in Fig 7. In addition to bisection, subjects were also asked to rate the degree of IM on a 1 to 5 point scale. In one condition where subjects reported minimal IM, bisection errors were in the same direction as background motion, a finding that replicates previous studies. Conversely, in the other condition where subjects reported IM present, bisection marks deviated in a direction opposite the background OKS.
Another study done by our group investigated eye movements while young healthy volunteers were asked to look at a stationary horizontal line superimposed on the OKS.119 The participants were not to bisect the line but only to observe the line. The results showed that fixations occurred in the opposite direction to the background movement-i.e., in the same direction as the IM. Normally, people look at (foveate) the area of the environment to which they are attending. In the presence of IM, their attention is therefore directed toward the portion of the line that appears to be on the leading side. More recently, we published another study in which normal subjects performed bisection under the influence of illusory motion.120 This study replicated and extended the results of Na et al.118 such that leftward MB induced a rightward bias, and vice versa in healthy volunteers. This study also found that there is a relationship between the magnitude of IM and the degree of bias. Future studies are needed to investigate whether older healthy subjects and patients with neglect are also affected by IM.
It has been repeatedly confirmed that OKS has treatment effect for hemispatial neglect. Dependent variables in these studies were line bisection performances, position sense, and even motor weakness of left hand. However, underlying mechanisms for this treatment effect has not been fully elucidated.
Results of many studies suggested that normally three sensory inputs (visual, proprioceptive, and vestibular input) are integrated at a central level to generate spatial coordinates necessary for personal and extrapersonal space explorations.99,100 In hemispatial neglect, errors occur at this coordinate generation systems, resulting in deviation of the spatial reference frame to the ipsilesional side. Pizzamiglio et al.109 suggested that the OKS such as a large moving background produces nystagmus characterized with a slow phase coherent with the movement and a quick phase going back to the initial fixation. The vestibular stimulation produces an attentional bias coherent with the direction of the slow phase of the nystagmus. Alternatively, OKS may have a treatment effect by transiently restoring this distorted spatial reference frame.111-114,116 More specifically, the positive effect of OKS in patients with spatial neglect is interpreted with a "correction" of the neural coordinate transformation process by producing asymmetric input at the sensory organs of the contributing channels.113
However, some behaviors shown by patients during OKS are not consistent with this hypothesis. When asked to bisect lines superimposed on a leftward moving background, patients with left neglect often place the bisection marks far too the left from the veridical midpoint rather than placing the marks accurately. If the distorted spatial reference frame is corrected by OKS, leftward OKS would make patients place the bisection marks accurately. Thus, it appears that leftward OKS does not correct the distorted reference frame but just shift the reference frame with the reference frame being distorted. In the same context, Bisiach et al.108 posit that OKS does not restore the distorted mental representation but "temporarily rectify the representational medium, or modulate attentional process within the disordered medium".
It has been suggested that the modulating effect of OKS on normal subjects and patients with neglect is associated with eye movement: slow phase of nystagmus. However, in Jeong et al's study,119 while watching stationary lines with the OKS background, no significant nystagmus was produced. Furthermore, if affected by the nystagmus, attentional bias of normal subjects occurred in the direction of rapid phase of the nystagmus. Therefore, attentional bias of subjects may be more associated with motion illusion rather than eye movements per se.
Since hemispatial neglect is a poor prognostic factor of functional outcome, any measures alleviating hemispatial neglect may have an important clinical implication from the patient's rehabilitation perspective. Lateralized or direction-specific stimulation of peripheral sensory systems such as left cold caloric stimulation and OKS with its slow component leftward can temporarily improve hemispatial neglect. According to recent functional MRI and PET studies, this improvement might result from the partial (re)activation of a distributed, multisensory vestibular network in the lesioned hemisphere, which is part of a system that codes ego-centered space. However, exact signal timing and directional selectivity of the network, especially in cases of OKS remains unknown. Left cold caloric stimulation basically activates bilateral distributed multisensory network, but preferentially activate the right hemisphere more than the left hemisphere. This hemispheric difference can explain why left cold caloric stimulation improves left-spaced neglect. However, functional activation studies on OKS have failed to show consistent results for hemispheric difference between leftward and rightward OKS. Therefore, future studies are needed to learn what processes participate in improving hemispatial neglect by OKS.
Figures and Tables
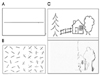 | Figure 1An illustration of left hemispatial neglect in right hemisphere stroke (A) Line bisection, (B) Modified Albert's line cancellation, (C) Copying of modified ogeden picture. |
 | Figure 2(A) Pathway for vestibulo-ocular reflex by left cold caloric stimulation. Cold water irrigation on left ear causes tonic deviation of eyeball to the left and rapid corrective eye movements toward the right. Thick lines are excited pathways and dotted lines are inhibited ones. (B) Central connection of vestibular system. Note that the vestibular apparatus is connected ipsilaterally with the spinal cord and cerebellum. Projections in the medial longitudinal fasciculus are both crossed and uncrossed. The projection to the thalamus and cerebral cortex is incompletely understood. III, oculomotor nuclei; IV, trochlear nuclei; VI, abducens nuclei; VIII, vestibular nuclei. |
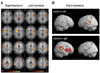 | Figure 3(A) Areas activated during caloric stimulation (warm water at 44℃) in the right ear of the right-handed healthy volunteers and in the left ear of those left-handed (group analysis; n=12; P<0.001; 15O-labelled H2O bolus, positron emission tomography). Activations were located in the anterior and posterior insula, the STG, the inferior frontal gyrus, the post-central gyrus, the IPL and the anterior cingulum. Note that the activations were more pronounced in right-handers during irrigation of the right ear in the right hemisphere and in left-handers during irrigation of the left ear in the left hemisphere. This indicates dominance of the non-dominant hemisphere in the processing of vestibular information. (B) Lateral views of the surfaces of both hemispheres showing activated areas during caloric stimulation of the right or left ear in right-handers in the superior temporal cortex, TPJ, insular cortex and inferior frontal cortex. Compared with the activation pattern during caloric irrigation of the right ear, caloric irrigation of the left ear led to activations which were smaller in both hemispheres and more frequently located within the ipsilateral left hemisphere. These results represent dominance of the ipsilateral vestibular pathways.49
|
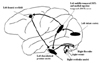 | Figure 4A hypothetical scheme for horizontal smooth pursuit. Primary visual cortex (V1) projects to the homologue of the middle temporal visual area (MT) that in humans lies at the temporal-occipital-parietal junction. MT projects to the homologue of the medial superior temporal visual area (MST) and also to the frontal eye field (FEF). MST also receives inputs from its contralateral counterpart. MST projects through the retrolenticular portion of the internal capsule and the posterior portion of the cerebral peduncle to the dorsolateral pontine nucleus (DLPN). The DLPN also receives inputs important for pursuit from the FEF; these inputs descend in the medial portion of the cerebral peduncle. The DLPN projects, mainly contralaterally, to the flocculus, paraflocculus, and ventral uvula of the cerebellum; projections also pass to the dorsal vermis. The flocculus projects to the ipsilateral vestibular nuclei (VN), which in turn project to the contralateral abducens nucleus. Dotted circles show structures on the opposite side. |
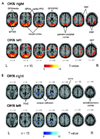 | Figure 5(A) OKN during rightward (upper row) and leftward (lower row) small-field visual stimulation vs. rest condition (stationary screen) in a group of 15 healthy right-handed volunteers elicited very similar bilateral activations of the visual cortex, which merged into the adjacent occipitotemporal (motion-sensitive area MT/V5) and parietooccipital areas including the parietal eye field (PEF) along the intraparietal sulcus. Additional activations were located nearly symmetrically in the anterior insular region and the adjacent parts of the inferior frontal gyri (GFi) as well as in different ocular motor structures such as the prefrontal cortex (PFC, GFm=middle frontal gyrus), frontal (FEF), and supplementary eye fields (SEF). For illustrative purposes, voxels above a threshold of P≤0.005 uncorrected are shown. (B) OKN during rightward (upper row) and leftward (lower row) small-field visual stimulation in a group of 15 healthy right-handed volunteers caused deactivations in the posterior corpus callosum which partly merged into adjacent parts of the posterior cingulate gyrus and optic radiation. Additional bilateral deactivations were found in the parieto-insular vestibular cortex (PIVC) in the posterior insula, in the central sulcus region (best attributed to the somatosensory cortex), and in the frontal-most and medial part of the right middle frontal gyrus (BA 8, GFm). For illustrative purposes, voxels above a threshold of P≤0.005 uncorrected are shown.92
|
 | Figure 6Exploratory scan paths of an exemplary patient with spatial neglect (left) and a healthy subject (right) while searching for a (non-existent) target in darkness with their heads fixed. The upper panel shows the patients' exploratory eye movements with no further stimulation; the lower panel is the result under left-sided vestibular stimulation (cold water irrigation). In the condition without stimulation, the neglect patient showed a bias of ocular exploration towards the right and neglect of the left, while symmetrical eye movements were observed under unilateral vestibular stimulation. The healthy subject showed exactly the opposite behavior, i.e. symmetrical search without stimulation and asymmetrical search under vestibular stimulation.84
|
References
1. Heilman K.M., Watson R.T., Valenstein E. Heilman K.M., Valenstein E, editors. Neglect and related disorders. Clinical Neuropsychology. 2003. 4th ed. New York: Oxford University Press;296–346.
2. Gainotti G, Messerli P, Tissot R. Qualitative analysis of unilateral spatial neglect in relation to laterality of cerebral lesions. J Neurol Neurosurg Psychiatry. 1972. 35:545–550.

3. Hier DB, Mondlock J, Caplan LR. Recovery of behavioral abnormalities after right hemisphere stroke. Neurology. 1983. 33:345–350.

4. Stone SP, Wilson B, Wroot A, Halligan PW, Lange LS, Marshall JC, Greenwood RJ, et al. The assessment of visuo-spatial neglect after acute stroke. J Neurol Neurosurg Psychiatry. 1991. 54:345–350.

6. Gauthier L, Dehaut F, Joanette Y. The bells test: a quantitative and qualitative test for visual neglect. Int Clin Neuropsychol. 1989. 11:49–54.
7. Ogden JA. Anterior-posterior interhemispheric differences in the loci of lesions producing visual hemineglect. Brain and Cognition. 1985. 4:59–75.

8. Halligan PW, Marshall JC, Wade DT. Visuospatial neglect: underlying factors and test sensitivity. Lancet. 1989. 2:908–911.

9. Denes G, Semenza C, Stoppa E, Lis A. Unilateral spatial neglect and recovery from hemiplegia: a follow-up study. Brain. 1982. 105:543–552.

10. Katz N, Hartman-Maeir A, Ring H, Soroker N. Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Arch Phys Med Rehabil. 1999. 80:379–384.

11. Karnath HO, Zihl J. Brandt T, Caplan LR, Dichgans J, Diener HC, Kennard C, editors. Disorders of spatial orientation. Neurological disorders: course and treatment. 2003. San Diego, CA: Academic Press;277–286.

12. Lawson IR. Visual-spatial neglect in lesions of the right cerebral hemisphere. A study in recovery. Neurology. 1962. 12:23–33.

13. Pizzamiglio L, Antonucci G, Judica A, Montenero P, Razzano C, Zoccolotti P. Cognitive rehabilitation of the hemineglect disorder in chronic patients with unilateral right brain damage. J Clin Exp Neuropsychol. 1992. 14:901–923.

14. Robertson IH, North N. Spatio-motor cueing in unilateral left neglect: the role of hemispace, hand and motor activation. Neuropsychologia. 1992. 30:553–563.

15. Liepert J. Transcranial magnetic stimulation in neurorehabilitation. Acta Neurochir Suppl. 2005. 93:71–74.

16. Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, et al. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature. 1998. 395:166–169.

17. Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 1966. 153:1544–1547.

18. Arai T, Ohi H, Sasaki H, Nobuto H, Tanaka K. Hemispatial sunglasses: effect on unilateral spatial neglect. Arch Phys Med Rehabil. 1997. 78:230–232.

19. Beis JM, Andre JM, Baumgarten A, Challier B. Eye patching in unilateral spatial neglect: efficacy of two methods. Arch Phys Med Rehabil. 1999. 80:71–76.

20. Na DL, Adair JC, Williamson DJ, Schwartz RL, Haws B, Heilman KM. Dissociation of sensory-attentional from motor-intentional neglect. J Neurol Neurosurg Psychiatry. 1998. 64:331–338.

21. Bankiewicz KS, Oldfield EH, Plunkett RJ, Schuette WH, Cogan DG, Hogan N, et al. Apparent unilateral visual neglect in MPTP-hemiparkinsonian monkeys is due to delayed initiation of motion. Brain Res. 1991. 541:98–102.

22. Fleet WS, Valenstein E, Watson RT, Heilman KM. Dopamine agonist therapy for neglect in humans. Neurology. 1987. 37:1765–1770.

23. Geminiani G, Bottini G, Sterzi R. Dopaminergic stimulation in unilateral neglect. J Neurol Neurosurg Psychiatry. 1998. 65:344–347.

24. Abzug C, Maeda M, Peterson BW, Wilson VJ. Cervical branching of lumbar vestibulo-spinal axons. J Physiol. 1974. 243:499–522.

25. Iwamoto Y, Perlmutter SI, Baker JF, Peterson BW. Spatial coordination by descending vestibular signals. 2. Response properties of medial and lateral vestibulospinal tract neurons in alert and decerebrate cats. Exp Brain Res. 1996. 108:85–100.
26. Nathan PW, Smith M, Deacon P. Vestibulospinal, reticulospinal and descending propriospinal nerve fibres in man. Brain. 1996. 119:1809–1833.

27. Nishiike S, Guldin WO, Baurle J. Corticofugal connections between the cerebral cortex and vestibular nuclei in rat. J Comp Neurol. 2000. 420:363–372.

28. Akbarian S, Grüsser O-J, Guldin WO. Corticofugal connections between the cerebral cortex and brainstem vestibular nuclei in the macaque monkey. J Comp Neurol. 1994. 339:421–437.

29. Fredrickson JM, Figge U, Scheid P, Kornhuber HH. Vestibular nerve projection to the cerebral cortex of the rhesus monkey. Exp Brain Res. 1966. 2:318–327.

30. Schwarz DWF, Fredrickson JM. Rhesus monkey vestibular cortex: a bimodal primary projection field. Science. 1971. 172:280–281.

31. Ödkvist LM, Schwarz DWF, Fredrickson JM, Hassler R. Projection of the vestibular nerve to the area 3a arm field in the squirrel monkey (Saimiri sciureus). Exp Brain Res. 1974. 21:97–105.

32. Büttner U, Buettner UW. Parietal cortex (2v) neuronal activity in the alert monkey during natural vestibular and OKS. Brain Res. 1978. 153:392–397.

33. Faugier-Grimaud S, Ventre J. Anatomic connections of inferior parietal cortex (Area 7) with subcortical structures related to vestibulo-ocular function in a monkey (Macaca fascicularis). J Comp Neurol. 1989. 280:1–14.

34. Grüsser OJ, Pause M, Schreiter U. Localization and responses of neurons in the parieto-insular cortex of awake monkeys (Macaca fascicularis). J Physiol (Lond). 1990. 430:537–557.

35. Grüsser OJ, Pause M, Schreiter U. Vestibular neurones in the parieto-insular cortex of monkeys (Macaca fascicularis): visual and neck receptor responses. J Physiol. 1990. 430:559–583.

36. Guldin WO, Grüsser OJ. Collard M, Jeannerod M, Christen Y, editors. The anatomy of the vestibular cortices of primates. Le cortex vestibulaire. 1996. Editions IRVINN. Paris: Ipsen;17–26.
37. Bremmer F, Klam F, Duhamel J-R, Hamed SB, Graf W. Visual-vestibular interactive responses in the macaque ventral intraparietal area (VIP). Eur J Neurosci. 2002. 16:1569–1586.

38. Klam F, Graf W. Vestibular signals of posterior parietal cortex neurons during active and passive head movements in macaque monkeys. Ann N Y Acad Sci. 2003. 1004:271–282.

39. Klam F, Graf W. Vestibular response kinematics in posterior parietal cortex neurons of macaque monkeys. Eur J Neurosci. 2003. 18:995–1010.

40. Ebata S, Sugiuchi Y, Izawa Y, Shinomiya K, Shinoda Y. Vestibular projection to the periarcuate cortex in the monkey. Neurosci Res. 2004. 49:55–68.

41. Schlack A, Sterbing-D'Angelo SJ, Hartung K, Hoffmann KP, Bremmer F. Multisensory space representations in the macaque ventral intraparietal area. J Neurosci. 2005. 25:4616–4625.

42. Bottini G, Sterzi R, Paulesu E, Vallar G, Cappa SF, Erminio F, et al. Identification of the central vestibular projections in man: a positron emission tomography activation study. Exp Brain Res. 1994. 99:164–169.

43. Bucher SF, Dieterich M, Wiesmann M, Weiss A, Zink R, Yousry T, et al. Cerebral functional MRI of vestibular, auditory, and nociceptive areas during galvanic stimulation. Ann Neurol. 1998. 44:120–125.

44. Lobel E, Kleine JF, Le Bihan D, Leroy-Willig A, Berthoz A. Functional MRI of galvanic vestibular stimulation. J Neurophysiol. 1998. 80:2699–2709.

45. Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J Neurophysiol. 2001. 85:886–899.

46. Bremmer F, Schlack A, Duhamel J-R, Graf W, Fink GR. Space coding in primate posterior parietal cortex. Neuroimage. 2001. 14:S46–S51.

47. Suzuki M, Kitano H, Ito R, Kitanishi T, Yazawa Y, Ogawa T, et al. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2001. 12:441–449.

48. Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, et al. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002. 17:1384–1393.

49. Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Brandt T, et al. Dominance for vestibular cortical function in the non-dominant hemisphere. Cerebral Cortex. 2003. 13:994–1007.

50. Emri M, Kisely M, Lengyel Z, Balkay L, Marian T, Miko L, et al. Cortical projection of peripheral vestibular signaling. J Neurophysiol. 2003. 89:2639–2646.

51. Stephan T, Deutschländer A, Nolte A, Schneider E, Wiesmann M, Brandt T, et al. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage. 2005. 26:721–732.

52. Wenzel R, Bartenstein P, Dieterich M, Danek A, Weindl A, Minoshima S, et al. Deactivation of human visual cortex during involuntary ocular oscillations. A PET activation study. Brain. 1996. 119:101–110.

53. Dieterich M, Bartenstein P, Spiegel S, Bense S, Schwaiger M, Brandt T. Thalamic infarctions cause side-specific suppression of vestibular cortex activations. Brain. 2005. 128:2052–2067.

54. Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988. 240:740–749.

55. Tusa RJ, Ungerleider L. Fiber pathways of cortical areas mediating smooth pursuit eye movements in monkeys. Ann Neurol. 1988. 23:174–183.

56. Ffytche DH, Guy CN, Zeki S. The parallel visual motion inputs into areas V1 and V5 of human cerebral cortex. Brain. 1995. 118:1375–1394.

57. Rodman HR, Gross CG, Albright TD. Afferent basis of visual response properties in area MT of the macaque. I. Effect of striate cortex removal. J Neurosci. 1989. 9:2033–2050.

58. Desimone R, Ungerleider LG. Multiple visual areas in the causal superior temporal sulcus of the macaque. J Comp Neurol. 1986. 248:164–189.

59. Komatsu H, Wurtz RH. Relation of cerebral areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol. 1988. 60:580–603.

60. Maunsell JHR, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol. 1983. 49:1127–1147.

61. Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal area (MT). J Neurosci. 1988. 8:2201–2211.

62. Dürsteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol. 1988. 60:940–965.

63. Tootell RB, Taylor JB. Anatomical evidence for MT and additional cortical visual areas in humans. Cerebral Cortex. 1995. 5:39–55.

64. Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSI. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1997. 11:641–649.

65. Barton JJS, Sharpe JA, Raymond JE. Retinotopic and directional defects in motion discrimination in humans with cerebral lesions. Ann Neurol. 1995. 37:665–675.

66. Barton JJS, Sharpe JA, Raymond JE. Directional defects in pursuit and motion perception in humans with unilateral cerebral lesions. Brain. 1996. 119:1535–1550.

67. Zihl J, Von Crammon D, Mai N. Selective disturbance of movement vision after bilateral nrain damage. Brain. 1983. 106:313–340.

68. Morrow MJ, Sharpe JA. Cerebral hemispheric localization of smooth pursuit asymmetry. Neurology. 1990. 40:284–292.

69. Thurston SE, Leigh RJ, Crawford T, Thompson A, Kennard C. Two distinct deficits of visual tracking caused by unilateral lesions of cerebral cortex in man. Ann Neurol. 1988. 23:266–273.

70. Felleman DJ, Van Essen DC. Distributed hierarchial processing in the primate cerebral cortex. Cereb Cortex. 1991. 1:1–47.
71. Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation od retinal from extraretinal inputs. J Neurophysiol. 1988. 60:604–620.

72. Duffy CJ, Wurtz RH. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large field stimuli. J Neurophysiol. 1991. 65:1329–1345.

73. Graziano MS, Anderson RA, Snowden RJ. Tuning of MST neurons to spiral motions. J Neurosci. 1994. 14:54–57.

74. Barton JJS, Simpson T, Kiriakopoulos E, Stewart C, Crawley A, Gauthrie B, et al. Functional MRI of lateral occipitotemporal cortex during pursuit and motion perception. Ann Neurol. 1996. 40:387–398.

75. Scalaidhe SP, Albright TD, Rodman HR, Gross CG. Effect of superior temporal polysensory area lesions on eye movements in the macaque monkey. J Neurophysiol. 1995. 73:1–19.

76. Brandt SA, Dale AM, Wenzel R, Culham JC, Mendola JD, Tootel RBH. Sensory, motor and attentional components of eye movement induced cortex activation revealed by fMRI. Soc Neurosci Abstr. 1997. 23:2223.
77. Glickstein M, Cohen JL, Dixon B, Gibson A, Hollins M, Labossiere E, Robinson F, et al. Corticopontine visual projections in macaque monkeys. J Comp Neurol. 1980. 190:209–229.

78. May RJ, Keller EL, Suzuki DA. Smooth-pursuit eye movement deficits with chemical lesions in the dorsolateral pontine nucleus of the monkey. J Neurophysiol. 1988. 59:952–977.

79. Mustari MJ, Fuchs AF, Wallman J. Response properties of dorsolateral pontine units during smooth pursuit in the rhesus macaque. J Neurophysiol. 1988. 60:664–686.

80. Glickstein M, Gerrits N, Kralj-hans I, Mercier B, Stein J, Voogd J. Visual pontocerebellar projections in the macaque. J Comp Neurol. 1994. 349:51–72.

81. Brodal P. Further observations on the cerebellar projections from the pontine nuclei and the nucleus reticularis tegmenti pontis in the rhesus monkey. J Comp Neurol. 1982. 204:44–55.

82. Bucher SF, Dieterich M, Seelos KC, Brandt T. Sensorimotor cerebral activation during optokinetic nystagmus. A functional MRI study. Neurology. 1997. 49:1370–1377.

83. Dieterich M, Bucher SF, Seelos KC, Brandt T. Horizontal or vertical OKS activates visual motion-sensitive, ocular motor and vestibular cortex areas with right hemispheric dominance. An fMRI study. Brain. 1998. 121:1479–1495.

84. Galati G, Pappata S, Pantano P, Lenzi GL, Samson Y, Pizzamiglio L. Cortical control of optokinetic nystagmus in humans: a positron emission tomography study. Exp Brain Res. 1999. 126:149–159.

85. Rosano C, Krisky CM, Welling JS, Eddy WF, Luna B, Thulborn KR, Sweeny JA, et al. Pursuit and saccadic eye movement subregions in human frontal eye fields: a high-resolution fMRI investigation. Cereb Cortex. 2002. 12:107–115.

86. Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996. 34:475–483.

87. Pierrot-Deseilligny C, Milea D, Müri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004. 17:17–25.

88. Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, et al. A large-scale distributed network for covert spatial attention. Further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999. 122:1093–1106.
89. Gitelman DR, Parrish TB, Friston KJ, Mesulam MM. Functional anatomy of visual search: regional segregations within the frontal eye fields and effective connectivity of the superior colliculus. Neuroimage. 2002. 15:970–982.

90. Weber B, Schwarz U, Kneifel S, Treyer V, Buck A. Hierarchical visual processing is dependent on the oculomotor system. Neuroreport. 2000. 11:241–247.

91. Bense S, Janusch B, Schlindwein P, Bauermann T, Vucurevic G, Brandt T, et al. Direction-dependent visual cortex activation during horizontal OKS (fMRI study). Hum Brain Mapp. 2005. 08. 03. [Epub ahead of print].
92. Gottlieb JP, MacAvoy MG, Bruce CJ. Neuronal responses related to smooth pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. J Neurophysiol. 1994. 72:1634–1653.

93. Hoffmann K-P, Bremmer F, Thiele A, Distler C. Directional asymmetry of neurons in cortical areas MT and MST projecting to the NOT-DTN in macaques. J Neurophysiol. 2002. 87:2113–2123.

94. Huk AC, Ress D, Heeger DJ. Neuronal basis of the motion aftereffect reconsidered. Neuron. 2001. 32:161–172.

95. Brandt T, Bartenstein P, Janek A, Dieterich M. Reciprocal inhibitory visual-vestibular interaction: visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain. 1998. 121:1749–1758.

96. Abderhalden E. Lehrbuch der Physiologie in Vorlesungen. 1926. Bd. 3. Berlin: Urban & Schwarzenberg.
97. Jung R. Bergmann Gv, Frey W, Schwiegk H, editors. Neurophysiologische Untersuchungsmethoden. Handbuch der Inneren Medizin. 1953. Bd. V. Berlin: Springer;1206–1420.

98. Karnath H-O, Himmelbach M, Perenin M-T. Neglect-like behaviour in healthy subjects: dissociation of space exploration and goal-directed pointing following vestibular stimulation. Exp Brain Res. 2003. 153:231–238.
100. Cappa SF, Sterzi R, Vallar G, Bisiach E. Remission of hemineglect and anosognosia during vestibular stimulation. Neuropsychologia. 1987. 25:775–782.

101. Geminiani G, Bottini G. Mental representation and temporary recovery from unilateral neglect after vestibular stimulation. J Neurol Neurosurg Psychiatry. 1992. 55:332–333.

102. Rode G, Charles N, Perenin MT, Vighetto A, Trillet M, Aimard G. Partial remission of hemiplegia and somatoparaphrenia through vestibular stimulation in a case of unilateral neglect. Cortex. 1992. 28:203–208.

103. Adair JC, Na DL, Schwartz RL, Heilman KM. Caloric stimulation in neglect: evaluation of response as a function of neglect type. J Int Neuropsychol Soc. 2003. 9:983–988.

104. Storrie-Baker HJ, Segalowitz SJ, Black SE, McLean JA, Sullivan N. Improvement of hemispatial neglect with cold-water calorics: an electrophysiological test of the arousal hypothesis of neglect. J Int Neuropsychol Soc. 1997. 3:394–402.

105. Karnath HO. Subjective body orientation in neglect and the interactive contribution of neck muscle proprioception and vestibular stimulation. Brain. 1994. 117:1001–1012.

107. Mattingley JB, Bradshaw JL, Bradshaw JA. Horizontal visual motion modulates focal attention in left unilateral spatial neglect. J Neurol Neurosurg Psychiatry. 1994. 57:1228–1235.

108. Bisiach E, Pizzamiglio L, Nico D, Antonucci G. Beyond unilateral neglect. Brain. 1996. 119:851–857.

109. Pizzamiglio L, Frasca R, Guariglia C, Incoccia C, Antonucci G. Effect of OKS in patients with visual neglect. Cortex. 1990. 26:535–540.
110. Zoccolotti P, Guariglia C, Pizzamiglio L, Judica A, Razzano C, Pantano P. Good recovery in visual scanning in a patient with persistent anosognosia. Int J Neurosci. 1992. 63:93–104.

111. Vallar G, Antonucci G, Guariglia C, Pizzamiglio L. Deficits of position sense, unilateral neglect and OKS. Neuropsychologia. 1993. 31:1191–1200.

112. Vallar G, Guariglia C, Magnotti L, Pizzamiglio L. OKS affects both vertical and horizontal deficits of position sense in unilateral neglect. Cortex. 1995. 31:669–683.

113. Karnath HO. OKS influences the disturbed perception of body orientation in spatial neglect. J Neurol Neurosurg Psychiatry. 1996. 60:217–220.

114. Vallar G, Guariglia C, Nico D, Pizzamiglio L. Motor deficits and OKS in patients with left hemineglect. Neurology. 1997. 49:1364–1370.

115. Vallar G, Guariglia C, Magnotti L, Pizzamiglio L. Dissociation between position sense and visual-spatial components of hemineglect through a specific rehabilitation treatment. J Clin Exp Neuropsychol. 1997. 19:763–771.

116. Kerkhoff G, Schindler I, Keller I, Marquardt C. Visual background motion reduces size distortion in spatial neglect. Neuroreport. 1999. 10:319–323.

117. Pizzamiglio L, Fasotti L, Jehkonen M, Antonucci G, Magnotti L, Boelen D, et al. The use of OKS in rehabilitation of the hemineglect disorder. Cortex. 2004. 40:441–450.

118. Na DL, Son Y, Kim CH, Lee BH, Shon YM, Lee KJ, et al. Effect of background motion on line bisection performance in normal subjects. Cortex. 2002. 38:787–796.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download