Abstract
Background and Purpose
Methods
Results
Acknowledgements
References
Supplementary Materials
Fig. 2
Effects estimates for the association between the use of AEDs and CA-IMT. AEDs: antiepileptic drugs, CA-IMT: carotid artery intima-media thickness, MD: mean difference.
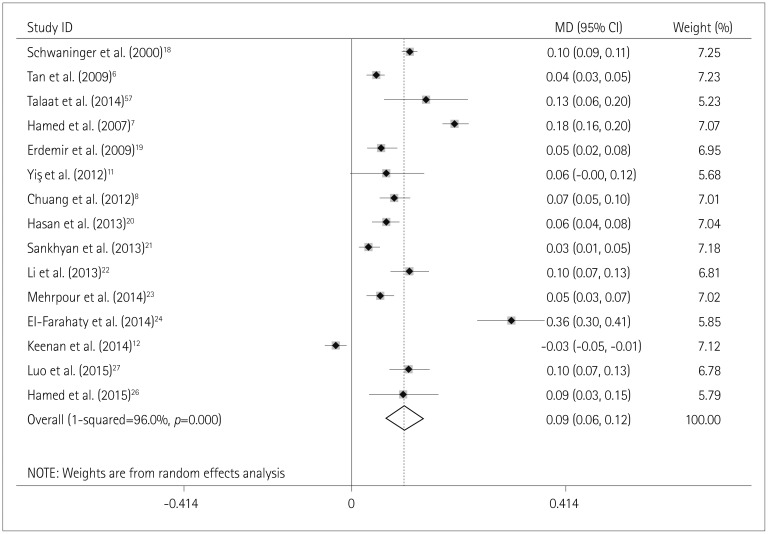
Fig. 3
Funnel plot for studies of the use of AEDs and CA-IMT. AEDs: antiepileptic drugs, CA-IMT: carotid artery intima-media thickness, MD: mean difference.
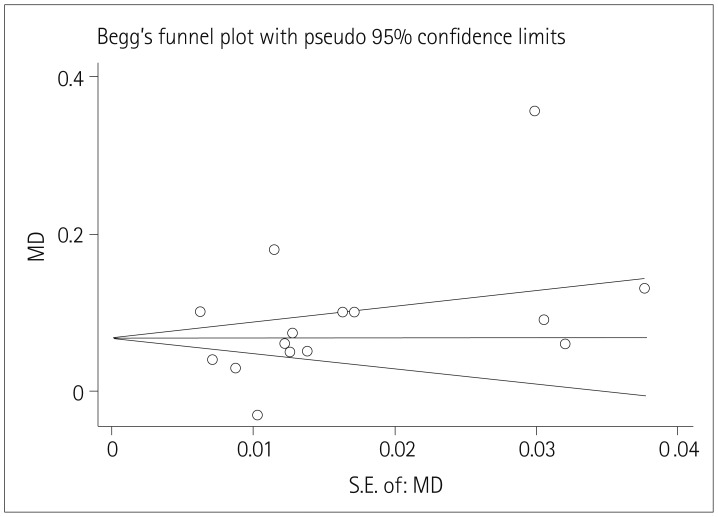
Fig. 4
Effects estimates for the association between the use of CBZ and CA-IMT. CA-IMT: carotid artery intima-media thickness, CBZ: carbamazepine, MD: mean difference.
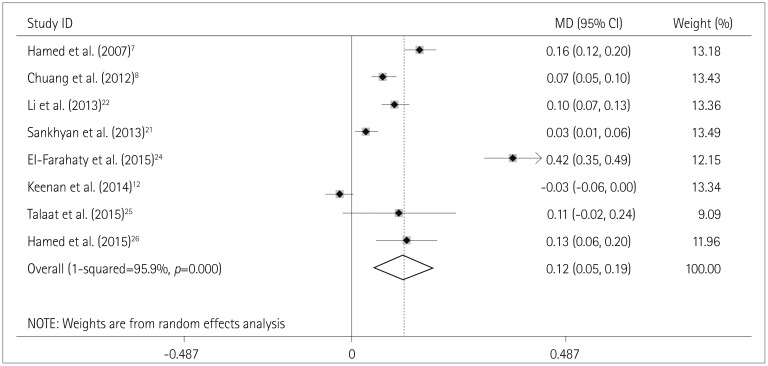
Fig. 5
Effects estimates for the association between the use of VPA and CA-IMT. CA-IMT: carotid artery intima-media thickness, MD: mean difference, VPA: valproic acid.
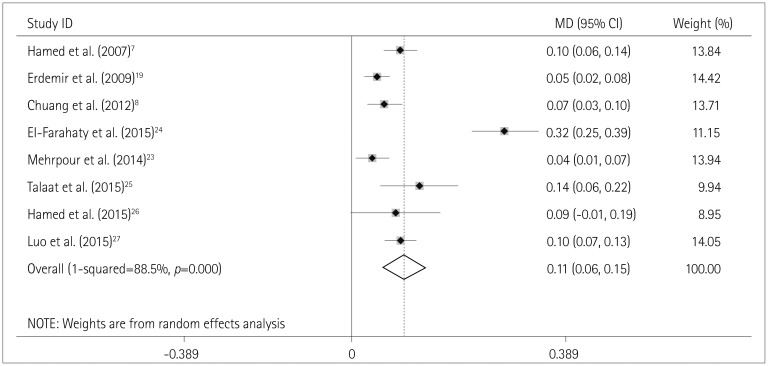
Table 1
Newcastle-Ottawa scale for assessing the quality each study
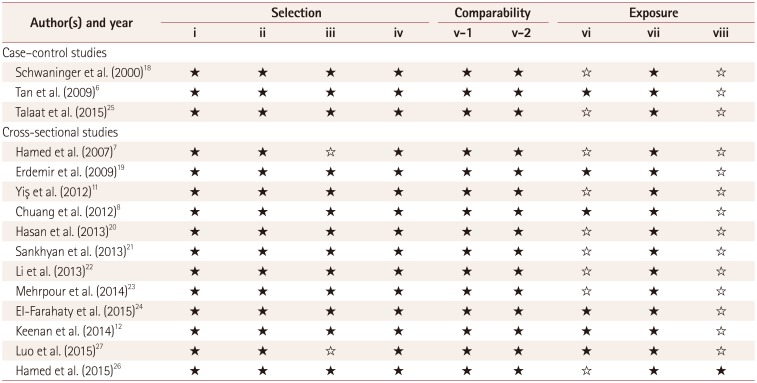
| Author(s) and year | Selection | Comparability | Exposure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| i | ii | iii | iv | v-1 | v-2 | vi | vii | viii | |
| Case-control studies | |||||||||
| Schwaninger et al. (2000)18 | ★ | ★ | ★ | ★ | ★ | ★ | ☆ | ★ | ☆ |
| Tan et al. (2009)6 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ☆ |
| Talaat et al. (2015)25 | ★ | ★ | ★ | ★ | ★ | ★ | ☆ | ★ | ☆ |
| Cross-sectional studies | |||||||||
| Hamed et al. (2007)7 | ★ | ★ | ☆ | ★ | ★ | ★ | ☆ | ★ | ☆ |
| Erdemir et al. (2009)19 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ☆ |
| Yis¸ et al. (2012)11 | ★ | ★ | ★ | ★ | ★ | ★ | ☆ | ★ | ☆ |
| Chuang et al. (2012)8 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ☆ |
| Hasan et al. (2013)20 | ★ | ★ | ★ | ★ | ★ | ★ | ☆ | ★ | ☆ |
| Sankhyan et al. (2013)21 | ★ | ★ | ★ | ★ | ★ | ★ | ☆ | ★ | ☆ |
| Li et al. (2013)22 | ★ | ★ | ★ | ★ | ★ | ★ | ☆ | ★ | ☆ |
| Mehrpour et al. (2014)23 | ★ | ★ | ★ | ★ | ★ | ★ | ☆ | ★ | ☆ |
| EI-Farahaty et al. (2015)24 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ☆ |
| Keenan et al. (2014)12 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ☆ |
| Luo et al. (2015)27 | ★ | ★ | ☆ | ★ | ★ | ★ | ★ | ★ | ☆ |
| Hamed et al. (2015)26 | ★ | ★ | ★ | ★ | ★ | ★ | ☆ | ★ | ★ |
★Represents good, ☆Represents poor. Selection: i) Adequacy of the case definition, ii) Representativeness of the cases, iii) Selection of controls, iv) Definition of controls. Comparability: v) Comparability of cases and controls based on the design or analysis method [v-1) Controls for the most important factor and v-2) Controls for any additional factor]. Exposure: vi) Ascertainment of exposure, vii) Same method used to ascertain cases and controls, viii) Nonresponse rate.
Table 2
Characteristics of the studies included in the final analysis
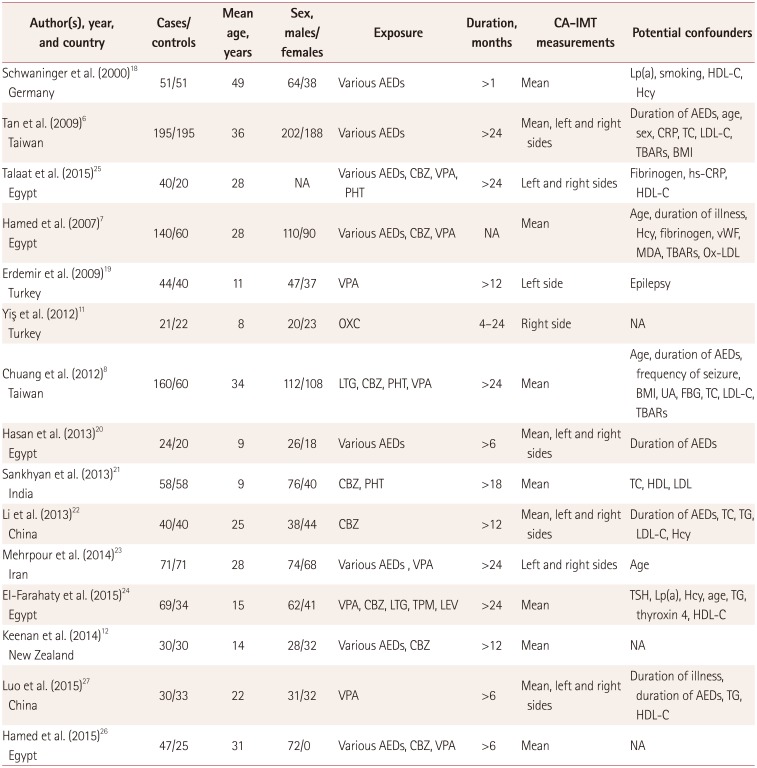
| Author(s), year, and country | Cases/controls | Mean age, years | Sex, males/ females | Exposure | Duration, months | CA-IMT measurements | Potential confounders |
|---|---|---|---|---|---|---|---|
|
Schwaninger et al. (2000)18 Germany |
51/51 | 49 | 64/38 | Various AEDs | >1 | Mean | Lp(a), smoking, HDL-C, Hcy |
|
Tan et al. (2009)6 Taiwan |
195/195 | 36 | 202/188 | Various AEDs | >24 | Mean, left and right sides | Duration of AEDs, age, sex, CRP, TC, LDL-C, TBARs, BMI |
|
Talaat et al. (2015)25 Egypt |
40/20 | 28 | NA | Various AEDs, CBZ, VPA, PHT | >24 | Left and right sides | Fibrinogen, hs-CRP, HDL-C |
|
Hamed et al. (2007)7 Egypt |
140/60 | 28 | 110/90 | Various AEDs, CBZ, VPA | NA | Mean | Age, duration of illness, Hcy, fibrinogen, vWF, MDA, TBARs, Ox-LDL |
|
Erdemir et al. (2009)19 Turkey |
44/40 | 11 | 47/37 | VPA | >12 | Left side | Epilepsy |
|
Yis¸ et al. (2012)11 Turkey |
21/22 | 8 | 20/23 | OXC | 4–24 | Right side | NA |
|
Chuang et al. (2012)8 Taiwan |
160/60 | 34 | 112/108 | LTG, CBZ, PHT, VPA | >24 | Mean | Age, duration of AEDs, frequency of seizure, BMI, UA, FBG, TC, LDL-C, TBARs |
|
Hasan et al. (2013)20 Egypt |
24/20 | 9 | 26/18 | Various AEDs | >6 | Mean, left and right sides | Duration of AEDs |
|
Sankhyan et al. (2013)21 India |
58/58 | 9 | 76/40 | CBZ, PHT | >18 | Mean | TC, HDL, LDL |
|
Li et al. (2013)22 China |
40/40 | 25 | 38/44 | CBZ | >12 | Mean, left and right sides | Duration of AEDs, TC, TG, LDL-C, Hcy |
|
Mehrpour et al. (2014)23 Iran |
71/71 | 28 | 74/68 | Various AEDs , VPA | >24 | Left and right sides | Age |
|
EI-Farahaty et al. (2015)24 Egypt |
69/34 | 15 | 62/41 | VPA, CBZ, LTG, TPM, LEV | >24 | Mean | TSH, Lp(a), Hcy, age, TG, thyroxin 4, HDL-C |
|
Keenan et al. (2014)12 New Zealand |
30/30 | 14 | 28/32 | Various AEDs, CBZ | >12 | Mean | NA |
|
Luo et al. (2015)27 China |
30/33 | 22 | 31/32 | VPA | >6 | Mean, left and right sides | Duration of illness, duration of AEDs, TG, HDL-C |
|
Hamed et al. (2015)26 Egypt |
47/25 | 31 | 72/0 | Various AEDs, CBZ, VPA | >6 | Mean | NA |
AEDs: antiepileptic drugs, BMI: body mass index, CA-IMT: carotid artery intima–media thickness, CBZ: carbamazepine, FBG: fasting blood glucose, Hcy: homocysteine, HDL-C: high-density lipoprotein cholesterol, PHT: phenytoin, LDL-C: low-density lipoprotein cholesterol, LEV: levetiracetam, Lp(a): lipoprotein a, LTG: lamotrigine, MDA: malondialdehyde, NA: not available, OXC: oxcarbazepine, Ox-LDL: oxidized low-density lipoprotein, TBARs: thiobabituric-acid-reactive substance, TC: total cholesterol, TG: triglyceride, TPM: topiramate, TSH: thyroid-stimulating hormone, UA: uric acid, VPA: valproic acid, vWF: von Willebrand Factor.
Table 3
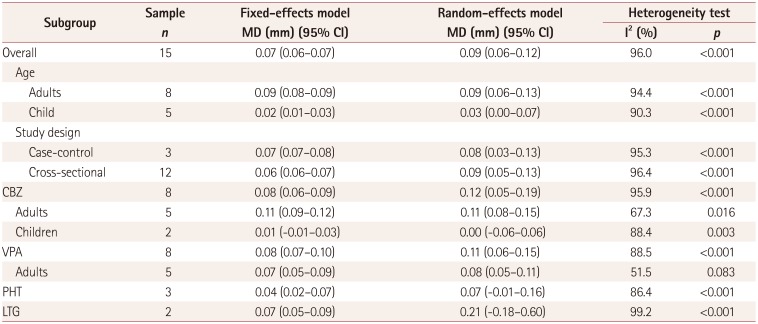
Subgroup analysis for the use of AEDs and CA-IMT




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download