Abstract
Background and Purpose
The objective of this study was to find a sensitive method for the early detection of diabetic polyneuropathy (DPN) and determine the relationship between the functions of somatic and autonomic small nerve fibers in DPN.
Methods
Patients with type 2 diabetes mellitus and DPN based on clinical symptoms, signs, intraepidermal nerve fiber density (IENFD), and findings in the quantitative sudomotor axon reflex test (QSART) were enrolled retrospectively. Neurological examinations and nerve conduction studies were performed on all patients. Heart-rate variability during deep breathing (DB ratio) and the Valsalva maneuver (Valsalva ratio) were used to quantify the cardiovagal function. Patients were divided into two groups: 1) normal nerve conduction, defined as small-fiber neuropathy (SFN) and 2) abnormal nerve conduction, defined as mixed-fiber neuropathy.
Results
In total, 82 patients were enrolled (age: 60.7±10.7 years, mean±SD). A decreased IENFD was the most frequent abnormality across all of the patients, followed by abnormalities of the QSART and cardiovagal function. A decreased IENFD was more sensitive than the QSART, DB ratio, and Valsalva ratio for detecting diabetic SFN. The DB ratio was significantly correlated with the duration of diabetes mellitus and clinical symptoms and signs. There was no correlation between the IENFD and the findings of the QSART for the distal leg.
Diabetic polyneuropathy (DPN) is usually a sensory neuropathy, and it can cause painless injury and result in foot amputation.1 Many patients initially present with symptoms and signs of small-fiber neuropathy (SFN), and so a diagnostic tool for detecting the function of small nerve fibers is crucial for detecting DPN in the early stage. Various specialized modalities for testing SFN have been developed, including the intraepidermal nerve fiber density (IENFD), quantitative sensory testing (QST), sudomotor function test, nociceptive evoked potential, and microneurography.
Diagnostic criteria for SFN have been proposed based on retrospective data analyses.2 The IENFD was the single most sensitive method for detecting SFN, and the combination of the IENFD, QST, and a clinical examination showed higher sensitivity and specificity in diagnosing SFN.
Many studies have found the IENFD to be decreased in patients with impaired glucose tolerance as well as early DPN.34567 Recent guidelines have recommended the use of a skin biopsy as a standard for detecting SFN.8910 The quantitative sudomotor axon reflex test (QSART) provides a quantitative and validated assessment of postganglionic sudomotor function.11 Several studies have suggested that the findings of the QSART are frequently abnormal in clinically suspected SFN1112 and diabetic SFN.13 The QSART also plays an additive role in diagnosing SFN when combined with other tests.131415
Investigations of the association between autonomic and somatic measures in SFN have produced conflicting findings. One study found a significant association between sudomotor function as measured by the composite autonomic severity score and IENFD.16 They enrolled a small number of SFN patients with various etiologies. Other studies have found no overall correlation between the QSART volume and IENFD in SFN patients with various etiologies.1718 These conflicting results emphasize the need to perform further studies to determine the relationship between autonomic function and the IENFD in SFN. In addition, no study has compared the results obtained using the QSART and IENFD in DPN. DPN has a more specific and homogeneous etiology than SFN, and hence enrolling patients with DPN only could reduce the selection bias. The present study was designed to identify the relationship between the QSART and IENFD in DPN. We included heart-rate variability (HRV) because it has also been reported to be a sensitive test for detecting SFN.17
The objectives of this study were 1) to identify the most sensitive method among the IENFD, QSART, and HRV for diagnosing DPN and diabetic SFN and 2) to determine the relationship between the IENFD and the QSART, HRV, and clinical features in DPN.
We retrospectively reviewed the medical charts of patients with type 2 diabetes mellitus who were suspected of having DPN. Symptoms were assessed using the Neuropathy Symptom Score (NSS),19 and a clinical examination was performed using the Neuropathy Impairment Score (NIS).20 Specialized tests (IENFD and/or QSART) were used to detect the function of distal small nerve fibers. DPN was diagnosed if the patient had two or more abnormalities of the NSS, NIS, IENFD, and QSART. Patients with any other disorder that might cause polyneuropathy and those with a history of inherited neuropathy were excluded. The duration of diabetes mellitus, duration of symptoms, serum glucose level, and the most up-to-date HbA1c level were collected. This study was approved by our local Human Research Ethics Committee.
The NSS is measured using grouped subsets of motor, sensory, and autonomic questions, which are scored as present (1) or absent (0) by history-taking. NSS was regarded as abnormal if the summed score was higher than 1.19 The NIS comprises subsets of cranial nerve examination, motor examination, reflex tests, and sensory examination. Muscle weakness was scored from 0 (normal strength) to 4 (100% weakness), and tendon reflexes and sensory function were scored from 0 (normal) to 2 (absent). The NIS was regarded as abnormal if the summed score was higher than 2.20 Participants were also evaluated for vitamin B12 or folate deficiency, thyroid or other autoimmune disease, malignancy, and the use of neurotoxic drugs in order to rule out other possible causes of polyneuropathy.
Routine nerve conduction studies (NCS) were applied to all patients, including of the motor nerves (median, ulnar, peroneal, and tibial nerves) and the sensory nerves (median, ulnar, and sural nerves). Skin temperature was maintained at 32.0–34.0℃. The threshold for the presence of abnormal NCS findings was an abnormality (≥99th or ≤1st percentile) in any attribute of nerve conduction in two separate nerves, one of which must be the sural nerve.21 Patients with normal findings in NCS were regarded as having diabetic SFN, while those with abnormal findings were considered as having diabetic mixed-fiber neuropathy (MFN). The results of clinical, laboratory and electrophysiological tests were compared between SFN and MFN.
Two 3-mm punch biopsies were obtained at 10 cm above the lateral malleolus and 20 cm below the greater trochanter from each patient. Each skin biopsy specimen was fixed in a 2% paraformaldehyde-lysine-periodate solution and sectioned at a thickness of 50 µm using a freezing microtome. Immunostaining was performed using antibodies against Protein Gene Product 9.5 (Ultraclone, Wellow, UK) according to the standard method.22 The IENFD was analyzed visually by a single rater in all patients according to the guideline of the European Federation of the Neurological Societies.923 The IENFD in the distal leg was defined as abnormal if it was lower than 0.05 quantile of its normal value.24
The QSART was implemented using the Q-Sweat system (WR Medical, Minnesota, MN, USA). Iontophoresis with acetylcholine was used to stimulate postganglionic sudomotor nerves and induce sweat. The sweat produced was collected and quantified at four sites: 1) forearm (three-quarters of the distance from the ulnar epicondyle to the pisiform bone), 2) proximal leg (5 cm distal to the fibular head on the lateral side), 3) distal leg (5 cm proximal to the medial malleolus on the medial side), and 4) foot (over the extensor digitorum brevis muscle). Normal values were based on published normative data, and we regarded the result at any site as abnormal if the total sweat volume was lower than the normal value.25
Cardiovagal function was evaluated using the beat-to-beat HRV to deep breathing and the Valsalva maneuver. The HRV during deep breathing (DB ratio) was measured based on electrocardiogram recordings during six smooth breathing cycles over 1 minute made using the Finapres® device (FMS, Enschede, the Netherlands). The result was expressed as the mean difference between the maximum and minimum heart rates. The heart-rate response to the Valsalva maneuver (Valsalva ratio) was determined by asking the patient to blow into a mouthpiece connected to a sphygmomanometer and holding it at a pressure of 40 mm Hg for 15 seconds while recording the electrocardiogram. The Valsalva ratio was recorded as the ratio of the longest R-R interval after the maneuver to the shortest R-R interval during the maneuver.
SPSS (version 20.0, IBM Corp., Armonk, NY, USA) was used for all statistical analyses. The independent t-test was applied to linear variables, and the chi-square test was applied to categorical variables. Correlation and logistic regression analyses were performed to assess relationships among the tests. A probability value of p<0.05 was considered statistically significant.
In total, 82 patients were enrolled in this study (age: 60.7±10.7 years, mean±SD). The deep breathing and Valsalva ratios were obtained for 72 and 70 patients, respectively. The IENFD was determined for 60 patients, while QSART was performed for 42 patients. Forty of the 82 patients were classified as MFN according to results of NCS, while 42 were classified as SFN.
The clinical characteristics and laboratory findings of all participants and comparisons between SFN and MFN are presented in Table 1. The age, sex, disease duration, and symptom duration did not differ significantly between SFN and MFN. The serum glucose level, HbA1c level, NSS, and NIS were significantly higher in MFN than in SFN.
Results for the IENFD, HRV, and QSART are summarized in Table 2. The IENFD in the distal leg was lower in MFN than in SFN. Abnormality of the DB ratio was more common in MFN than in SFN. Abnormality of the IENFD was the most frequent abnormality in all patients, followed by abnormalities of the DB ratio, QSART, and Valsalva ratio. In patients with SFN, abnormality of the IENFD was again the most frequent abnormality, followed by abnormalities of the QSART, Valsalva ratio, and DB ratio.
The DB ratio was negatively correlated with disease duration, NSS, and NIS, and positively correlated with the Valsalva ratio. There was no correlation between the HRV and QSART or IENFD. The QSART volume and IENFD in the distal leg were not correlated even in subgroup analyses of the abnormal-IENFD or abnormal-QSART group.
The IENFD was more sensitive than the HRV and QSART for detecting the early stage of DPN. There was no relationship between the IENFD and QSART in the distal leg in DPN. The DB ratio was significantly correlated with the disease duration, symptoms, and findings of clinical examinations. However, there was no correlation between the IENFD, HRV, and QSART.
Since the first report of using the IENFD to investigate SFN,26 analyzing the IENFD has become a standard method for diagnosing SFN and diabetic SFN.923 The IENFD was decreased in patients with DPN in the present study, occurring in 80% of all patients and in 75.8% of SFN patients. The frequency of a decreased IENFD was similar to that in reported previously by Shun et al.7 (81%), who investigated the IENFD and QST in patients with type 2 diabetes and concluded that small-fiber sensory neuropathy presenting with a reduced IENFD was a major manifestation of type 2 diabetes mellitus. Reductions in the IENFD have also been reported in patients with impaired glucose tolerance6 and in diabetic patients with normal findings in NCS.45 Løseth et al.4 compared the IENFD and QST in diabetic patients with normal findings in NCS, and reported that the IENFD was more sensitive for detecting SFN than QST in diabetic patients with neuropathic symptoms. However, the present study is the first to compare the IENFD and QSART in type 2 diabetes mellitus, and the IENFD was found to be more sensitive than the QSART in diagnosing diabetic SFN and DPN.
The relationship between the IENFD and sudomotor function in SFN remains to be determined. Thaisetthawatkul et al.18 found no association between the IENFD and parameters of the QSART in SFN with various etiologies. They also found no association between an autonomic reflex screen and the QSART, IENFD, QST, pain symptoms, or abnormal pinprick sensations. Those authors suggested that autonomic and sensory outcomes are independent measures of distal SFN. On the other hand, Novak27 reported that the IENFD was correlated with the electrochemical skin conductance measured using the Sudoscan device (Impeto Medical, Paris, France), which is a derivative current produced by sweat chloride ions in response to applied stimulus. Forty-eight out of the 82 patients with SFN in that study were diagnosed as having idiopathic SFN, while the others had various SFN etiologies. We found no correlation between the IENFD and QSART in the distal leg in patients with type 2 diabetes mellitus. This discrepancy might be due to heterogeneity of the subjects or study designs, and so we enrolled patients with DPN only. We found no relationship between the IENFD and QSART even in subgroup analyses of the abnormal-IENFD group or abnormal-QSART group. The IENFD combined with other tests for small nerve fibers such as the QSART might increase the sensitivity in diagnosing diabetic SFN.
The DB ratio was correlated with disease duration, symptoms, and findings of clinical examinations in this study, in agreement with the results of a previous study.17 However, we could not find any correlation among IENFD, QSART, heart rate variability, and disease duration. A decreased IENFD and QSART abnormality were the earliest signs of DPN. They were already abnormal before advanced DPN, resulting in no association among the IENFD, QSART, and disease duration. In contrast, the DB ratio was correlated with disease duration, and so it could be useful as a progression marker of DPN.
This study was subject to several limitations. First, it had a retrospective design, and so selection bias might have been present when enrolling the subjects. Second, tests (IENFD, QSART, and HRV) for detecting SFN were not performed for all patients. In particular, the total number of QSART studies equaled only half the number of participants, which could also have introduced selection bias. Moreover, the number of cases of SFN was too small to allow the diagnostic sensitivity to be compared. Future prospective studies are needed to compare the diagnostic sensitivity of the IENFD and QSART in diabetic SFN. Finally, we used published normal data for the IENFD instead of our own normal controls, which constitutes another limitation of this study.
One strength of this study is that we enrolled patients with type 2 diabetes mellitus only, resulting in the included subjects being more homogeneous than in previous studies. To the best of our knowledge, this is the first study that has compared the IENFD and QSART in type 2 diabetes mellitus.
In conclusion, the IENFD was a more sensitive method than the QSART for detecting the early stage of DPN. The degree of involvement of the somatic small nerve fibers and sudomotor nerve fibers was independent in DPN.
References
1. Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999; 22:1029–1035. PMID: 10388962.

2. Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912–1925. PMID: 18524793.

3. Ragé M, Van Acker N, Knaapen MW, Timmers M, Streffer J, Hermans MP, et al. Asymptomatic small fiber neuropathy in diabetes mellitus: investigations with intraepidermal nerve fiber density, quantitative sensory testing and laser-evoked potentials. J Neurol. 2011; 258:1852–1864. PMID: 21472496.

4. Løseth S, Stålberg E, Jorde R, Mellgren SI. Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol. 2008; 255:1197–1202. PMID: 18574618.

5. Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle Nerve. 2007; 35:591–598. PMID: 17221881.

6. Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology. 2001; 57:1701–1704. PMID: 11706115.

7. Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain. 2004; 127:1593–1605. PMID: 15128619.

8. England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, et al. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R. 2009; 1:14–22. PMID: 19627868.
9. Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, et al. European federation of neurological societies/peripheral nerve society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European federation of neurological societies and the peripheral nerve society. Eur J Neurol. 2010; 17:903–912. e44–e49. PMID: 20642627.
10. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010; 33:2285–2293. PMID: 20876709.

11. Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006; 34:57–61. PMID: 16718689.

12. Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve. 1992; 15:661–665. PMID: 1324425.

13. Kamel JT, Vogrin SJ, Knight-Sadler RJ, Willems NK, Seiderer L, Cook MJ, et al. Combining cutaneous silent periods with quantitative sudomotor axon reflex testing in the assessment of diabetic small fiber neuropathy. Clin Neurophysiol. 2015; 126:1047–1053. PMID: 25449560.

14. Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN. Contribution of QSART to the diagnosis of small fiber neuropathy. Muscle Nerve. 2013; 48:883–888. PMID: 23649502.

15. Killian JM, Smyth S, Guerra R, Adhikari I, Harati Y. Comparison of sudomotor and sensory nerve testing in painful sensory neuropathies. J Clin Neuromuscul Dis. 2011; 12:138–142. PMID: 21321492.

16. Singer W, Spies JM, McArthur J, Low J, Griffin JW, Nickander KK, et al. Prospective evaluation of somatic and autonomic small fibers in selected autonomic neuropathies. Neurology. 2004; 62:612–618. PMID: 14981179.

17. Novak V, Freimer ML, Kissel JT, Sahenk Z, Periquet IM, Nash SM, et al. Autonomic impairment in painful neuropathy. Neurology. 2001; 56:861–868. PMID: 11294922.

18. Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN. Autonomic evaluation is independent of somatic evaluation for small fiber neuropathy. J Neurol Sci. 2014; 344:51–54. PMID: 24972819.

19. Dyck PJ. Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle Nerve. 1988; 11:21–32. PMID: 3277049.

20. Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O’Brien PC. Variables influencing neuropathic endpoints: the Rochester Diabetic Neuropathy Study of Healthy Subjects. Neurology. 1995; 45:1115–1121. PMID: 7783874.

21. England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005; 64:199–207. PMID: 15668414.

22. McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998; 55:1513–1520. PMID: 9865794.
23. Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005; 12:747–758. PMID: 16190912.

24. Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010; 15:202–207. PMID: 21040142.

25. Sletten DM, Weigand SD, Low PA. Relationship of Q-sweat to quantitative sudomotor axon reflex test (QSART) volumes. Muscle Nerve. 2010; 41:240–246. PMID: 19768767.

26. Kennedy WR, Said G. Sensory nerves in skin: answers about painful feet? Neurology. 1999; 53:1614–1615. PMID: 10563599.
27. Novak P. Electrochemical skin conductance correlates with skin nerve fiber density. Front Aging Neurosci. 2016; 8:199. PMID: 27605912.

Table 1
Clinical characteristics and laboratory findings of the participants
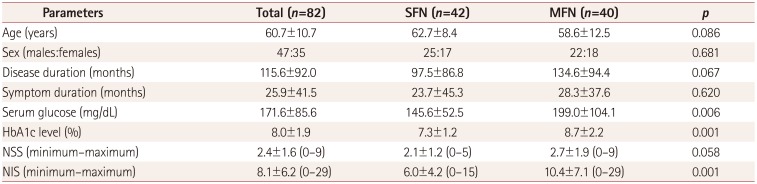
Table 2
Results for the IENFD, HRV, and QSART
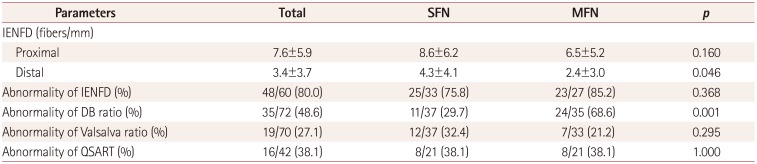
Data are mean±SD or n (%) values.
DB ratio: heart-rate variability during deep breathing, HRV: heart-rate variability, IENFD: intraepidermal nerve fiber density, MFN: mixed-fiber neuropathy, QSART: quantitative sudomotor axon reflex test, SFN: small-fiber neuropathy, Valsalva ratio: Valsalva maneuver.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download