1. Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003; 4:101–119. PMID:
14592341.
2. Earley CJ. Clinical practice. Restless legs syndrome. N Engl J Med. 2003; 348:2103–2109. PMID:
12761367.
3. Etgen T, Draganski B, Ilg C, Schröder M, Geisler P, Hajak G, et al. Bilateral thalamic gray matter changes in patients with restless legs syndrome. Neuroimage. 2005; 24:1242–1247. PMID:
15670702.

4. Unrath A, Juengling FD, Schork M, Kassubek J. Cortical grey matter alterations in idiopathic restless legs syndrome: an optimized voxel-based morphometry study. Mov Disord. 2007; 22:1751–1756. PMID:
17566123.

5. Quatrale R, Manconi M, Gastaldo E, Eleopra R, Tugnoli V, Tola MR, et al. Neurophysiological study of corticomotor pathways in restless legs syndrome. Clin Neurophysiol. 2003; 114:1638–1645. PMID:
12948792.

6. Scalise A, Cadore IP, Gigli GL. Motor cortex excitability in restless legs syndrome. Sleep Med. 2004; 5:393–396. PMID:
15222998.

7. Schober T, Wenzel K, Feichtinger M, Schwingenschuh P, Strebel A, Krausz G, et al. Restless legs syndrome: changes of induced electroencephalographic beta oscillations-an ERD/ERS study. Sleep. 2004; 27:147–150. PMID:
14998252.

8. Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS). Sleep Med. 2004; 5:385–391. PMID:
15222997.

9. Nardone R, Ausserer H, Bratti A, Covi M, Lochner P, Marth R, et al. Cabergoline reverses cortical hyperexcitability in patients with restless legs syndrome. Acta Neurol Scand. 2006; 114:244–249. PMID:
16942543.

10. Scalise A, Pittaro-Cadore I, Janes F, Marinig R, Gigli GL. Changes of cortical excitability after dopaminergic treatment in restless legs syndrome. Sleep Med. 2010; 11:75–81. PMID:
19595629.

11. Hornyak M, Feige B, Voderholzer U, Riemann D. Spectral analysis of sleep EEG in patients with restless legs syndrome. Clin Neurophysiol. 2005; 116:1265–1272. PMID:
15978488.

12. Stam CJ, Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys. 2007; 1:3. PMID:
17908336.

13. Ferri R, Rundo F, Bruni O, Terzano MG, Stam CJ. Small-world network organization of functional connectivity of EEG slow-wave activity during sleep. Clin Neurophysiol. 2007; 118:449–456. PMID:
17174148.

14. Spoormaker VI, Schröter MS, Gleiser PM, Andrade KC, Dresler M, Wehrle R, et al. Development of a large-scale functional brain network during human non-rapid eye movement sleep. J Neurosci. 2010; 30:11379–11387. PMID:
20739559.

15. Leistedt SJ, Coumans N, Dumont M, Lanquart JP, Stam CJ, Linkowski P. Altered sleep brain functional connectivity in acutely depressed patients. Hum Brain Mapp. 2009; 30:2207–2219. PMID:
18937282.

16. Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, Vourkas M, et al. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res. 2006; 87:60–66. PMID:
16875801.

17. Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P. Small-world networks and functional connectivity in Alzheimer's disease. Cereb Cortex. 2007; 17:92–99. PMID:
16452642.

18. Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014; 15:860–873. PMID:
25023924.
19. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991; 14:540–545. PMID:
1798888.

20. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001; 2:297–307. PMID:
11438246.

21. Sung HM, Kim JB, Ahn HN, Park YN, Bai DS, Lee SH. A study on the reliability and the validity of Korean version of the Beck Depression Inventory-II (BDI-II). J Korean Soc Biol Ther Psychiatry. 2008; 14:201–212.
22. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28:193–213. PMID:
2748771.

23. Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003; 4:121–132. PMID:
14592342.
24. Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester: American Academy of Sleep Medicine;2007.
25. Zucconi M, Ferri R, Allen R, Baier PC, Bruni O, Chokroverty S, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2006; 7:175–183. PMID:
16459136.

26. Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin Neurophysiol. 2006; 117:348–368. PMID:
16356767.

27. Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust. 1967; 15:70–73.

28. Mormann F, Lehnertz K, David P, Elger CE. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Physica D: Nonlinear Phenomena. 2000; 144:358–369.

29. Sporns O. Graph theory methods for the analysis of neural connectivity patterns. In : Kötter R, editor. Neuroscience Databases. edition: 2003. p. 171–185.
30. Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics. 2004; 2:145–162. PMID:
15319512.

31. Humphries MD, Gurney K. Network ‘small-world-ness’: a quantitative method for determining canonical network equivalence. PLoS One. 2008; 3:e0002051. PMID:
18446219.

32. Choi JW, Jang KM, Jung KY, Kim MS, Kim KH. Reduced theta-band power and phase synchrony during explicit verbal memory tasks in female, non-clinical individuals with schizotypal traits. PLoS One. 2016; 11:e0148272. PMID:
26840071.

33. Scalise A, Pittaro-Cadore I, Golob EJ, Gigli GL. Absence of postexercise and delayed facilitation of motor cortex excitability in restless legs syndrome: evidence of altered cortical plasticity? Sleep. 2006; 29:770–775. PMID:
16796215.
34. Tergau F, Wischer S, Paulus W. Motor system excitability in patients with restless legs syndrome. Neurology. 1999; 52:1060–1063. PMID:
10102429.

35. Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003; 62:143–150. PMID:
14638388.

36. Perrett SP, Dudek SM, Eagleman D, Montague PR, Friedlander MJ. LTD induction in adult visual cortex: role of stimulus timing and inhibition. J Neurosci. 2001; 21:2308–2319. PMID:
11264306.

37. Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981; 51:483–495. PMID:
6165548.

38. Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS One. 2007; 2:e276. PMID:
17342210.

39. Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004; 430:78–81. PMID:
15184907.

40. Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998; 393:440–442. PMID:
9623998.

41. Genzel L, Kroes MC, Dresler M, Battaglia FP. Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends Neurosci. 2014; 37:10–19. PMID:
24210928.

42. Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006; 9:1169–1176. PMID:
16936722.

43. Jung KY. Cognition in restless legs syndrome. J Sleep Med. 2015; 12:1–6.

44. Jung KY, Koo YS, Kim BJ, Ko D, Lee GT, Kim KH, et al. Electrophysiologic disturbances during daytime in patients with restless legs syndrome: further evidence of cognitive dysfunction? Sleep Med. 2011; 12:416–421. PMID:
21377421.

45. Kim SM, Choi JW, Lee C, Lee BU, Koo YS, Kim KH, et al. Working memory deficit in patients with restless legs syndrome: an event-related potential study. Sleep Med. 2014; 15:808–815. PMID:
24891078.

46. Pearson VE, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS). Sleep Med. 2006; 7:25–30. PMID:
16198145.

47. Choi JW, Ko D, Lee GT, Jung KY, Kim KH. Reduced neural synchrony in patients with restless legs syndrome during a visual oddball task. PLoS One. 2012; 7:e42312. PMID:
22848757.

48. Montplaisir J, Nicolas A, Denesle R, Gomez-Mancilla B. Restless legs syndrome improved by pramipexole: a double-blind randomized trial. Neurology. 1999; 52:938–943. PMID:
10102409.

49. Silber MH, Girish M, Izurieta R. Pramipexole in the management of restless legs syndrome: an extended study. Sleep. 2003; 26:819–821. PMID:
14655914.

50. Kallweit U, Khatami R, Pizza F, Mathis J, Bassetti CL. Dopaminergic treatment in idiopathic restless legs syndrome: effects on subjective sleepiness. Clin Neuropharmacol. 2010; 33:276–278. PMID:
21060282.
51. Jung KY, Kim SM, Song JY, Lee BU, Lee C, Lee SK, et al. Sternberg working memory performance following treatment with pramipexole in patients with moderate-to-severe restless legs syndrome. Sleep Med. 2015; 16:703–708. PMID:
25934537.

52. Saletu M, Anderer P, Saletu-Zyhlarz G, Hauer C, Saletu B. Acute placebo-controlled sleep laboratory studies and clinical follow-up with pramipexole in restless legs syndrome. Eur Arch Psychiatry Clin Neurosci. 2002; 252:185–194. PMID:
12242580.

53. Rizzo V, Aricò I, Mastroeni C, Morgante F, Liotta G, Girlanda P, et al. Dopamine agonists restore cortical plasticity in patients with idiopathic restless legs syndrome. Mov Disord. 2009; 24:710–715. PMID:
19117337.

54. Leemburg S, Vyazovskiy VV, Olcese U, Bassetti CL, Tononi G, Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci U S A. 2010; 107:15939–15944. PMID:
20696898.

55. Göttlich M, Münte TF, Heldmann M, Kasten M, Hagenah J, Krämer UM. Altered resting state brain networks in Parkinson's disease. PLoS One. 2013; 8:e77336. PMID:
24204812.

56. Luo CY, Guo XY, Song W, Chen Q, Cao B, Yang J, et al. Functional connectome assessed using graph theory in drug-naive Parkinson's disease. J Neurol. 2015; 262:1557–1567. PMID:
25929663.

57. Olde Dubbelink KT, Hillebrand A, Stoffers D, Deijen JB, Twisk JW, Stam CJ, et al. Disrupted brain network topology in Parkinson's disease: a longitudinal magnetoencephalography study. Brain. 2014; 137:197–207. PMID:
24271324.

58. Skidmore F, Korenkevych D, Liu Y, He G, Bullmore E, Pardalos PM. Connectivity brain networks based on wavelet correlation analysis in Parkinson fMRI data. Neurosci Lett. 2011; 499:47–51. PMID:
21624430.

59. Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007; 3:e17. PMID:
17274684.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


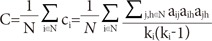

 XML Download
XML Download