1. Lampe AK, Bushby KM. Collagen VI related muscle disorders. J Med Genet. 2005; 42:673–685. PMID:
16141002.

2. Bönnemann CG. The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol. 2011; 7:379–390. PMID:
21691338.

3. Foley AR, Quijano-Roy S, Collins J, Straub V, McCallum M, Deconinck N, et al. Natural history of pulmonary function in collagen VI-related myopathies. Brain. 2013; 136:3625–3633. PMID:
24271325.

4. Briñas L, Richard P, Quijano-Roy S, Gartioux C, Ledeuil C, Lacène E, et al. Early onset collagen VI myopathies: genetic and clinical correlations. Ann Neurol. 2010; 68:511–520. PMID:
20976770.

5. Bethlem J, Wijngaarden GK. Benign myopathy, with autosomal dominant inheritance. A report on three pedigrees. Brain. 1976; 99:91–100. PMID:
963533.
6. Suh BC, Choi YC, Kim SM, Choi BO, Shim DS, Lee DH, et al. A family of Bethlem myopathy. J Korean Neurol Assoc. 2006; 24:614–617.
7. Park Y, Park MS, Sung DH, Sohn JY, Ki CS, Kim DH. Ullrich congenital muscular dystrophy possibly related with COL6A1 p.Gly302Arg variant. Ann Rehabil Med. 2014; 38:292–296. PMID:
24855628.

8. Park YE, Kim TH, Kim HS, Kim DS. A case of sporadic Ullrich congenital muscular dystrophy caused by a COL6A1 mutation. Ann Clin Neurophysiol. 2010; 12:27–31.
9. Chae JH, Vasta V, Cho A, Lim BC, Zhang Q, Eun SH, et al. Utility of next generation sequencing in genetic diagnosis of early onset neuromuscular disorders. J Med Genet. 2015; 52:208–216. PMID:
25635128.

10. Park HJ, Choi YC, Kim SM, Kim SH, Hong YB, Yoon BR, et al. Molecular genetic diagnosis of a Bethlem myopathy family with an autosomal-dominant COL6A1 mutation, as evidenced by exome sequencing. J Clin Neurol. 2015; 11:183–187. PMID:
25749816.
11. Park HJ, Jang H, Kim JH, Lee JH, Shin HY, Kim SM, et al. Discovery of pathogenic variants in a large Korean cohort of inherited muscular disorders. Clin Genet. 2017; 91:403–410. PMID:
27363342.

12. Fu J, Zheng YM, Jin SQ, Yi JF, Liu XJ, Lyn H, et al. “Target” and “Sandwich” signs in thigh muscles have high diagnostic values for collagen VI-related myopathies. Chin Med J (Engl). 2016; 129:1811–1816. PMID:
27453230.

13. Okada M, Kawahara G, Noguchi S, Sugie K, Murayama K, Nonaka I, et al. Primary collagen VI deficiency is the second most common congenital muscular dystrophy in Japan. Neurology. 2007; 69:1035–1042. PMID:
17785673.

14. Pace RA, Peat RA, Baker NL, Zamurs L, Mörgelin M, Irving M, et al. Collagen VI glycine mutations: perturbed assembly and a spectrum of clinical severity. Ann Neurol. 2008; 64:294–303. PMID:
18825676.

15. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424. PMID:
25741868.

16. Lampe AK, Dunn DM, von Niederhausern AC, Hamil C, Aoyagi A, Laval SH, et al. Automated genomic sequence analysis of the three collagen VI genes: applications to Ullrich congenital muscular dystrophy and Bethlem myopathy. J Med Genet. 2005; 42:108–120. PMID:
15689448.

17. Lucioli S, Giusti B, Mercuri E, Vanegas OC, Lucarini L, Pietroni V, et al. Detection of common and private mutations in the COL6A1 gene of patients with Bethlem myopathy. Neurology. 2005; 64:1931–1937. PMID:
15955946.

18. Vanegas OC, Zhang RZ, Sabatelli P, Lattanzi G, Bencivenga P, Giusti B, et al. Novel COL6A1 splicing mutation in a family affected by mild Bethlem myopathy. Muscle Nerve. 2002; 25:513–519. PMID:
11932968.

19. Yonekawa T, Nishino I. Ullrich congenital muscular dystrophy: clinicopathological features, natural history and pathomechanism(s). J Neurol Neurosurg Psychiatry. 2015; 86:280–287. PMID:
24938411.

20. Giusti B, Lucarini L, Pietroni V, Lucioli S, Bandinelli B, Sabatelli P, et al. Dominant and recessive COL6A1 mutations in Ullrich scleroatonic muscular dystrophy. Ann Neurol. 2005; 58:400–410. PMID:
16130093.

21. Pan TC, Zhang RZ, Sudano DG, Marie SK, Bönnemann CG, Chu ML. New molecular mechanism for Ullrich congenital muscular dystrophy: a heterozygous in-frame deletion in the COL6A1 gene causes a severe phenotype. Am J Hum Genet. 2003; 73:355–369. PMID:
12840783.

22. Baker NL, Mörgelin M, Pace RA, Peat RA, Adams NE, Gardner RJ, et al. Molecular consequences of dominant Bethlem myopathy collagen VI mutations. Ann Neurol. 2007; 62:390–405. PMID:
17886299.

23. Pepe G, Giusti B, Bertini E, Brunelli T, Saitta B, Comeglio P, et al. A heterozygous splice site mutation in COL6A1 leading to an in-frame deletion of the alpha1(VI) collagen chain in an italian family affected by bethlem myopathy. Biochem Biophys Res Commun. 1999; 258:802–807. PMID:
10329467.
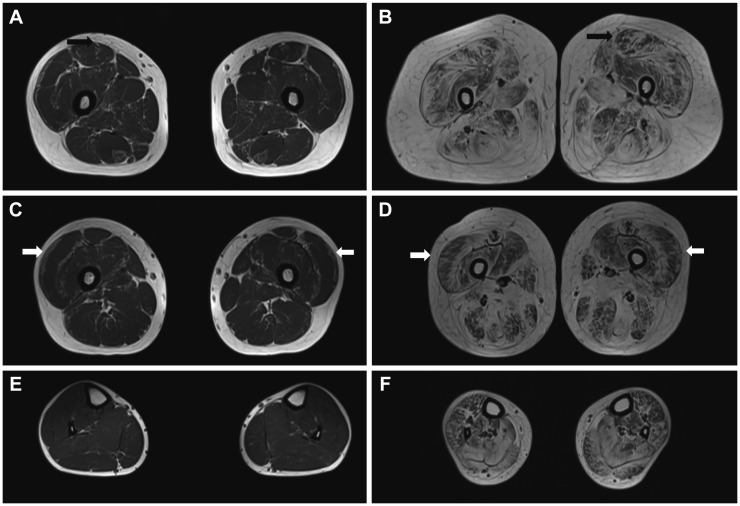
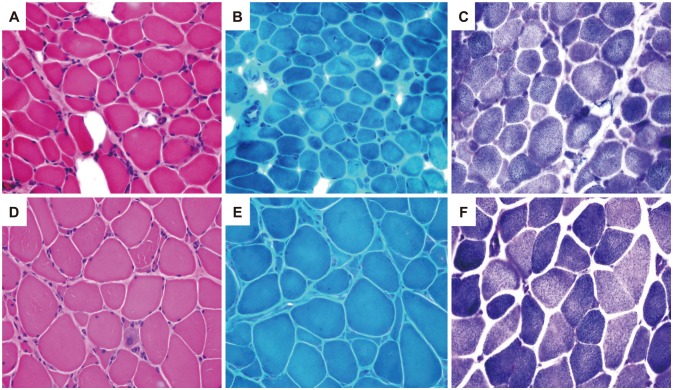
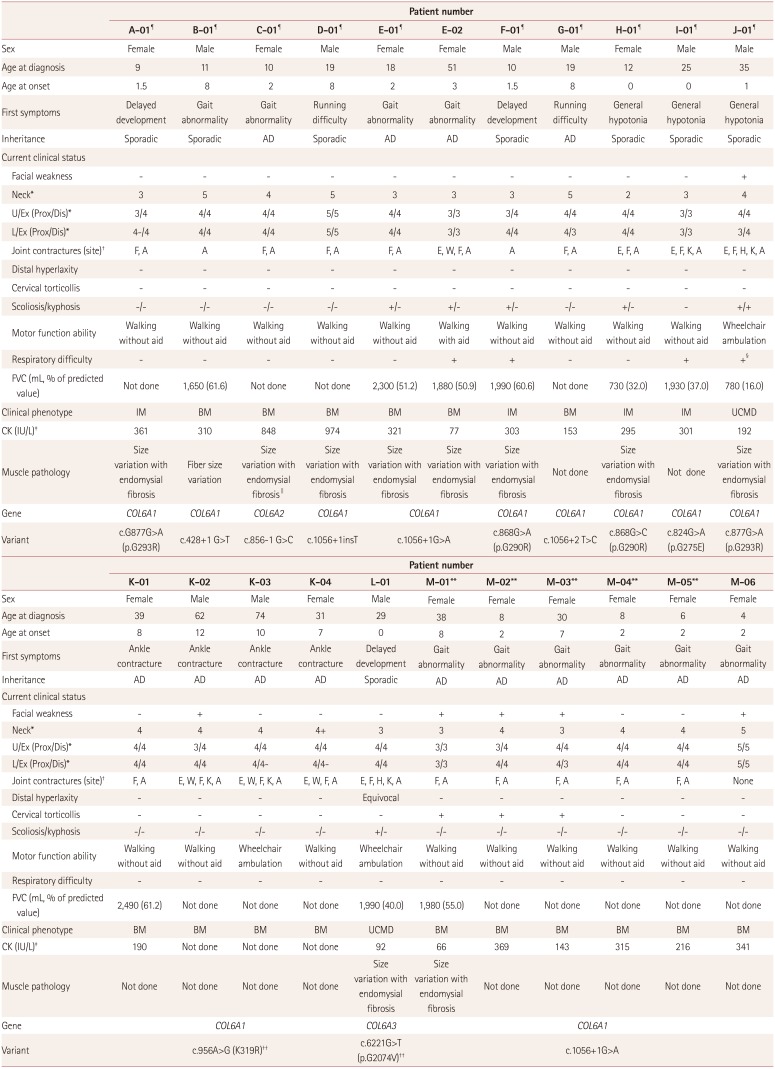
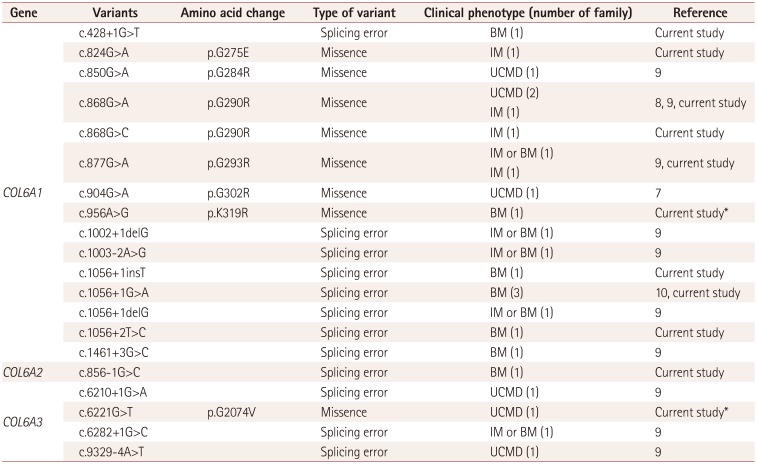




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download