Abstract
Background and Purpose
Upper respiratory infection (URI), including influenza, may exacerbate the symptoms of myasthenia gravis (MG), which is an autoimmune disease that causes muscle weakness. There is also concern that the influenza vaccine may trigger or worsen autoimmune diseases. The objective of this study was to determine the impacts of influenza infection and vaccination on symptom severity in MG patients.
Methods
Patients diagnosed with MG were enrolled from 10 university-affiliated hospitals between March and August 2015. Subjects completed a questionnaire at the first routine follow-up visit after enrolling in the study. The patient history was obtained to determine whether a URI had been experienced during the previous winter, if an influenza vaccination had been administered before the previous winter, and whether their MG symptoms were exacerbated during or following either a URI or vaccination. Influenza-like illness (ILI) was defined and differentiated from the common cold as a fever of ≥38℃ accompanied by a cough and/or a sore throat.
Results
Of the 258 enrolled patients [aged 54.1±15.2 years (mean±SD), 112 men, and 185 with generalized MG], 133 (51.6%) had received an influenza vaccination and 121 (46.9%) had experienced a common cold (96 patients) or ILI (25 patients) during the analysis period. MG symptoms were aggravated in 10 (40%) patients after ILI, whereas only 2 (1.5%) experienced aggravation following influenza vaccination. The rate of symptom aggravation was significantly higher in patients experiencing an ILI (10/25, 40%) than in those with the common cold (15/96, 15.6%, p=0.006).
It is known that upper respiratory infections (URIs) can exacerbate the symptoms of myasthenia gravis (MG) and, in some cases, can precipitate a myasthenic crisis.12 Annual influenza vaccines are recommended by the Center for Disease Control (CDC) as the first and most important preventive step against influenza infection and the potentially serious complications it can cause for patients with chronic neurological disorders.3 However, there is concern that the vaccines themselves may trigger or worsen the symptoms of certain immune-system-related diseases. In particular, there have been recent reports of an association between influenza vaccination and the risk of developing immune-mediated diseases such as Guillain-Barre syndrome.4567
Two previous studies that investigated the impact of influenza vaccination on patients with MG found no association between vaccination and patient safety, leading to the authors suggesting that seasonal influenza and H1N1 vaccines should be considered safe treatment options for MG patients.89 However, those studies focused mainly on the safety of influenza vaccination, and did not assess the risk of influenza-infection-associated exacerbation of MG symptoms.
In the present study, we investigated the impact of both influenza vaccination and influenza infection on symptom severity in MG patients.
Patients diagnosed with MG were consecutively enrolled from 10 university-affiliated hospitals across Korea between March and August 2015. The diagnosis of MG was based on clinical symptoms and a positive result in at least one of the following three laboratory diagnostic tests: neostigmine test, anti-acetylcholine receptor (AChR) antibody test, and repetitive nerve stimulation test.10 We collected demographic and clinical data including age, gender, disease duration, laboratory findings, history of thymoma or thymectomy, and current immunosuppressant regimen by reviewing the medical charts of all of the enrolled patients. All subjects were required to provide written informed consent, and the study was approved by the Institutional Review Board (IRB no. ED14307).
In order to assess clinical information regarding the history of URI and seasonal influenza vaccination, we developed a recall-based questionnaire and administered it to all of the included subjects at their first routine follow-up visit during the study period. Subjects were asked about their URI history–such as influenza-like illness (ILI) or the common cold–during the previous winter. Additionally, the questionnaire asked specifically if and when the patients had received an influenza vaccination during the previous year. To differentiate influenza-like symptoms after vaccination from actual influenza illness, questions regarding the dates of vaccine administration and the onset of ILI were included in the questionnaire. Finally, the subjects were asked if they experienced any exacerbation of their MG symptoms following a URI or influenza vaccination event. To evaluate the severity of worsening in MG symptoms, they were also asked to grade the degree of any exacerbation from 1 (representing “mildest possible exacerbation that did not interfere with daily activities”) to 10 (representing “worst possible exacerbation that required hospitalization”). MG symptom exacerbation was defined as an increase in the severity of at least one of the following objective symptoms: eyelid droop, double vision, difficulty holding the head up, facial muscle weakness affecting whistling or smiling, limb weakness, slurred or nasal speech, difficulty chewing or swallowing, or difficulty breathing. In accordance with the definition from the CDC, URIs were defined as symptoms related to URI that lasted for ≥1 day. ILI was differentiated from the common cold by a fever of ≥38℃ accompanied by a cough and/or a sore throat.11 Patients with a URI but not an ILI were classified as having the common cold.
Subjects were grouped according to whether or not they had received an influenza vaccination during the previous year, and we compared their demographic and clinical variables for effects on MG symptom severity. These variables were also compared between the ILI group and the common-cold group of patients who had a URI. Differences in clinical data between groups were assessed using the Mann-Whitney or chi-square test. We performed a logistic regression analysis in order to detect factors influencing MG symptom severity. A p value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
For this study, we enrolled a total of 258 patients [aged 54.1±15.2 years (mean±SD), 112 men, and 73 with ocular MG and 185 with generalized MG]. Positivity for the anti-AChR antibody was present in 220 (85.3%) of the subjects, and the median disease duration was 6.3 years (range=0.3–46.3 years). Of the 258 subjects, 184 (71.3%) had received prednisolone treatment and 117 (45.3%) had received other immunosuppressant drugs. In total, 111 subjects (43%) had undergone thymectomy, of which 32 were nonthymomatous MG cases, and the remaining 79 patients had thymoma. Overall, 133 (51.6%) patients had received an influenza vaccination during the previous year, and 121 (46.9%) had experienced a common cold (96 patients) or ILI (25 patients) during the previous winter. There were no cases with influenza-like symptoms or signs within 30 days after vaccination. Table 1 presents the clinical characteristics of the patients with and without vaccination.
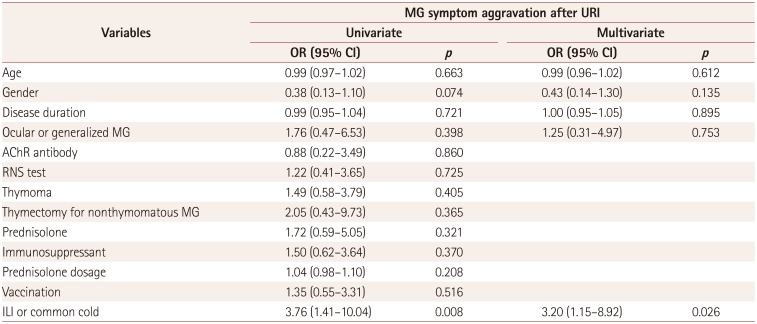
There were no statistically significant differences in MG clinical characteristics between the ILI and common-cold patient groups. However, the rate of MG symptom aggravation was significantly higher in ILI patients (10/25, 40.0%) than in the common-cold group (15/96, 15.6%, p=0.006), while the severity score for MG symptom aggravation did not differ significantly between these two groups (Table 2). A stepwise multivariate logistic regression analysis showed that ILI status was independently associated with MG exacerbation after adjusting for other confounding factors (odds ratio=3.20, 95% confidence interval=1.15–8.92, p=0.026) (Table 3).
MG symptom aggravation was found in 10 (40%) of 25 patients who had experienced an ILI during the previous winter, whereas only 2 of 133 patients (1.5% of 133) experienced symptom aggravation after receiving an influenza vaccination. The median severity score for symptom aggravation was 7.5 (range=3–9) in the ILI group and 6.5 (range=3–10) in the vaccinated group. Symptom aggravation occurred within 1 week after the ILI or influenza vaccination event in all cases.
We compared the impact of receiving a seasonal influenza vaccination or experiencing an influenza infection on symptom severity among MG patients based on their responses to our self-report questionnaire. We found that the risk of MG symptom exacerbation following seasonal influenza vaccination was very low (1.5%, 2/133), while experiencing an ILI was significantly associated with exacerbation of MG symptoms (40%, 10/25).
Previous studies have demonstrated that the influenza vaccine may sometimes trigger or exacerbate the symptoms of immune-mediated diseases such as Guillain-Barre syndrome, multiple sclerosis, and systemic lupus erythematosus.4567 However, the impact of influenza vaccination on patients with MG has not been investigated thoroughly. To our knowledge, only two studies have assessed the safety of influenza vaccination for MG patients.89 Those studies included 513 and 74 MG patients and found no increased symptom risk following seasonal or H1N1 influenza vaccination (although one of the studies did not consider mild symptom exacerbation in its assessment). Consistent with this previous work, we found that only 2 of the 133 patients (1.5%) who had received an influenza vaccination experienced exacerbation of MG symptoms. Together these results suggest that influenza vaccination is a safe option for MG patients.
In addition to assessing the safety of the influenza vaccine, the present study also determined the rate of influenza infection-related MG symptom exacerbation; this is an important potential effect to assess since it needs to be included in decisions about whether or not MG patients should receive influenza vaccination. Although several previous studies have shown that MG patients have an increased risk of experiencing a myasthenic crisis following an infection,12 there remains no evidence for a direct association between influenza infection and MG symptom severity. The present study conclusively found that ILI increased the probability of worsened MG symptoms (40% of patients, 10/25); this incidence rate was 2.5-fold higher than in MG patients who experienced a common cold (15.6%, 15/96). Furthermore, our multivariate analysis revealed that ILI status was the only independent factor associated with MG exacerbation after adjusting for other confounding factors. These findings suggest that influenza infection constitutes a specific and important risk factor for MG symptom exacerbation. It is therefore important to apply preventive measures for influenza infection to MG patients, since it is one of only a few modifiable risk factors for exacerbation of that disease.
Regarding vaccine efficacy, influenza vaccination did not confer protection for ILI since 11 (8.3%) of the 133 vaccinated patients had ILI compared with 14 (11.2%) of the unvaccinated patients. The reason for this suboptimal efficacy may be related to the vaccinated patients being older (60.0±13.6 years) than the unvaccinated patients (47.8±14.4 years), since being older can lead to an increased risk of influenza infection and to insufficient protection after vaccination. Another possible reason for low vaccine efficacy is the failure of the influenza vaccine during 2014–2015. The effectiveness of the influenza vaccine was lower (23%) during that season in the United States than in previous seasons due to a poor match between the vaccine strain and the circulating influenza viruses.12 A study in Korea also did not demonstrate any significant difference in the incidence of ILI between vaccinated and unvaccinated adults for the 2014–2015 season.13 Therefore, our results do not necessarily mean that influenza vaccination is ineffective in patients with MG.
As noted above, in the present study 8.3% of vaccinated and 11.2% of unvaccinated patients were found to have suffered from ILI. In Korea, 5–20% of the general population reportedly contract influenza each year,14 with the same incidence range reported in the United States.15 The World Health Organization has estimated that the influenza virus infects 10–20% of the global population each year.16 A study in Korea that assessed the incidence rate for ILI during the 2014–2015 season found that 11.3% of 265 adults had an ILI.13 Therefore, compared to the general population, the rate of influenza in our MG patients was not high.
In this study, only 51.6% (133/258) of the MG patients had received seasonal influenza vaccination during the previous year (a rate that is similar to that in a previously reported study). A previous study found that MG patients who elected to not receive influenza vaccination cited reasons such as fear of general and nonmyasthenic side effects (42.6%), fear of MG exacerbation (31.5%), and advice from physicians to not receive vaccination (14.8%).9 Although the present study did not assess the reasons why subjects did not receive a vaccination, its results may help reassure MG patients and their physicians that the influenza vaccine does not increase the risk of symptom exacerbation. MG patients do not currently have adequate guidelines for being vaccinated against influenza. At present, the CDC limits its recommendation to live attenuated vaccines, which are contraindicated for patients with chronic neurological disorders and patients taking immunosuppressant medications due to their higher risk of severe complications.317 Our findings provide important information for reassessing the guidelines relating to influenza vaccination in patients with MG.
This study was subject to some potential limitations that should be considered when interpreting the findings. First, we analyzed a relatively small number of subjects. Second, because most of the analyzed information was collected using a recall-based self-report questionnaire, it is possible that our results were affected by reporting bias. Third, the true rate of MG symptom exacerbation was difficult to estimate due to the results being self-reported, and it may be that pseudoexacerbation events were inappropriately included. Fourth, influenza infection and vaccination may have different impacts on patients with MG depending on the vaccine components and the immunosuppressant status of each patient. Thus, similar to two previous studies,89 our study may have been biased by these potential confounding factors. In order to minimize the effect of vaccine components, unlike a previous study which included 14 influenza seasons,8 we restricted our study to a single influenza season; that is, 2014–2015. Of course, we could not determine commercial product types for the influenza vaccine for each participant because our study had a recall-based design. However, almost all of the influenza vaccines used in Korea are trivalent unadjuvanted inactivated vaccines,18 with quadrivalent influenza vaccines not being available in Korea during the 2014–2015 season. Therefore, although additional vaccine components such as preservatives, adjuvants, and trace compounds can vary slightly between manufacturers, we assume that there are no differences in the main active components of vaccines. Many of the MG patients received immunosuppressant treatments that may have altered their immune responses to a vaccine. To minimize the effects of these biases, we attempted to identify information about prednisolone and other immunosuppressive treatments for each participant, and these factors were adjusted for in our analysis (e.g., for the results presented in Table 3). However, we did not examine the immunosuppressant status (e.g., dosage and duration) for all subjects, so our results are unlikely to be completely free from all potential sources of bias.
In conclusion, we found no significant association between seasonal influenza vaccination and MG symptom severity. This supports previous findings that the influenza vaccine is safe for MG patients. Additionally, we noted that ILI is associated with a substantially greater risk of symptom aggravation, and suggest that influenza vaccination should be recommended to MG patients as a preventive measure.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP; no. NRF-2015R1A5A7037674). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No financial disclosures were reported by the authors of this paper.
References
2. Kalita J, Kohat AK, Misra UK. Predictors of outcome of myasthenic crisis. Neurol Sci. 2014; 35:1109–1114. PMID: 24497206.

3. Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep. 2016; 65:1–54.

4. Agmon-Levin N, Kivity S, Shoenfeld Y. Influenza vaccine and autoimmunity. Isr Med Assoc J. 2009; 11:183–185. PMID: 19544711.
5. MartÍn Arias LH, Sanz R, Sáinz M, Treceño C, Carvajal A. Guillain-Barré syndrome and influenza vaccines: a meta-analysis. Vaccine. 2015; 33:3773–3778. PMID: 25999283.

6. Vellozzi C, Iqbal S, Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin Infect Dis. 2014; 58:1149–1155. PMID: 24415636.

7. McNicholas N, Chataway J. Relapse risk in patients with multiple sclerosis after H1N1 vaccination, with or without seasonal influenza vaccination. J Neurol. 2011; 258:1545–1547. PMID: 21336784.

8. Zinman L, Thoma J, Kwong JC, Kopp A, Stukel TA, Juurlink DN. Safety of influenza vaccination in patients with myasthenia gravis: a population-based study. Muscle Nerve. 2009; 40:947–951. PMID: 19902540.

9. Auriel E, Regev K, Dori A, Karni A. Safety of influenza and H1N1 vaccinations in patients with myasthenia gravis, and patient compliance. Muscle Nerve. 2011; 43:893–894. PMID: 21607970.

10. Hong YH, Kwon SB, Kim BJ, Kim BJ, Kim SH, Kim JK, et al. Prognosis of ocular myasthenia in Korea: a retrospective multicenter analysis of 202 patients. J Neurol Sci. 2008; 273:10–14. PMID: 18602121.

11. Cho S, Sohn CH, Jo MW, Shin SY, Lee JH, Ryoo SM, et al. Correlation between national influenza surveillance data and google trends in South Korea. PLoS One. 2013; 8:e81422. PMID: 24339927.

12. Flannery B, Clippard J, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, et al. Early estimates of seasonal influenza vaccine effectiveness-United States, January 2015. MMWR Morb Mortal Wkly Rep. 2015; 64:10–15. PMID: 25590680.
13. Yoo SY, Kim OS. Comparison of the incidence rate of influenza-like illness between an influenza-vaccinated group and unvaccinated group. J Korean Biol Nurs Sci. 2016; 18:110–117.

14. Song JY, Cheong HJ, Woo HJ, Wie SH, Lee JS, Chung MH, et al. Immunogenicity and safety of trivalent inactivated influenza vaccine: a randomized, double-blind, multi-center, phase 3 clinical trial in a vaccine-limited country. J Korean Med Sci. 2011; 26:191–195. PMID: 21286008.

15. Taylor C, Marathe A, Beckman R. Same influenza vaccination strategies but different outcomes across US cities? Int J Infect Dis. 2010; 14:e792–e795. PMID: 20643569.

16. Influenza vaccines. Wkly Epidemiol Rec. 2005; 80:279–287. PMID: 16171031.
17. Esposito S, Bruno C, Berardinelli A, Filosto M, Mongini T, Morandi L, et al. Vaccination recommendations for patients with neuromuscular disease. Vaccine. 2014; 32:5893–5900. PMID: 25223270.

18. Choi WS, Noh JY, Baek JH, Seo YB, Lee J, Song JY, et al. Suboptimal effectiveness of the 2011-2012 seasonal influenza vaccine in adult Korean populations. PLoS One. 2015; 10:e0098716. PMID: 25815717.

Table 1
Demographic, clinical, and laboratory characteristics of the study subjects
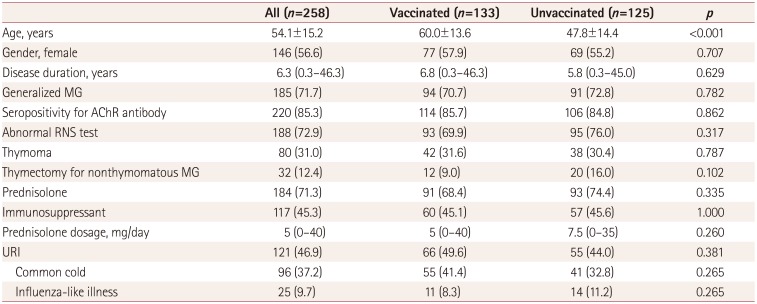
Table 2
Comparison of clinical data between the ILI and common-cold patient groups
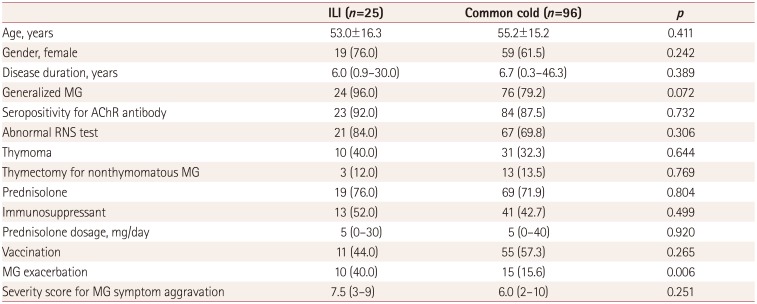
Table 3
Results from univariate and multivariate analyses of MG symptom aggravation after URI




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download