Abstract
Background and Purpose
Carotid endarterectomy (CEA) is performed to prevent cerebral infarction, but a common side effect is cerebral microinfarcts. This study aimed to identify the variables related to the production of microinfarcts during CEA as well as determine their association with delayed postoperative infarction.
Methods
This was a retrospective review of data collected prospectively from 548 patients who underwent CEA. The clinical characteristics of the patients and the incidence rates and causes of microinfarcts were analyzed. Microinfarcts were diagnosed by diffusion-weighted magnetic resonance imaging. The presence of delayed postoperative infarction was compared between microinfarct-positive and microinfarct-negative groups.
Results
In total, 76 (13.86%) patients were diagnosed with microinfarcts. Preoperative neurological symptoms were significantly related to the incidence of microinfarcts [odds ratio (OR)=2.93, 95% confidence interval (CI)=1.72–5.00, p<0.001]. Shunt insertion during CEA was the only significant procedure-related risk factor (OR=1.42, 95% CI=1.00–2.19, p=0.05). The presence of microinfarcts did not significantly increase the incidence of delayed postoperative infarction (p=0.204).
The effectiveness of carotid endarterectomy (CEA) in preventing strokes has been previously confirmed in both symptomatic and asymptomatic patients with carotid artery stenosis.12 However, one of the severe complications of CEA is stroke, which includes cerebral microinfarcts.34 Microinfarct lesions are tiny infarctions without any neurological symptoms that can be confirmed radiologically [generally by diffusion-weighted magnetic resonance imaging (DW-MRI)], and are mainly due to microembolisms. These lesions were found to be risk factors for cognitive impairment in the Cardiovascular Health Study Cognition Study5 and to be associated with a relatively poor prognosis for geriatric depression in a long-term follow-up study.6 The techniques used in carotid artery stenting (CAS) to treat carotid artery stenosis have evolved rapidly, and embolic protection filtering devices for reducing the incidence of microembolism, such as flow reversal systems and proximal arrest systems (Mo.Ma device, Medtronic Invatec, Frauenfeld, Switzerland), are currently in use.4 Similar to CAS-related procedures for reducing cerebral microembolisms during CEA, other preoperative methods such as gentler dissection techniques are available.7 However, the reported incidence of microembolism remains high, at 25–49%, and few studies have analyzed the risk factors for microembolism during CEA.89
Microinfarcts after CEA can result in poor outcomes, and microembolic signals are associated with an increased perioperative stroke risk1011 since this is associated with cognitive impairment and geriatric depression. The present study therefore aimed to elucidate the risk factors for microinfarcts after CEA in order to facilitate attempts to reduce its incidence. Additionally, we compared the infarction-free rate between microinfarct and no-microinfarct groups to assess its potential association with delayed cerebral infarction.
This study was conducted as a retrospective review of medical data collected prospectively from 556 patients who underwent CEA at our institution between January 2009 and December 2014. The study was approved by the Institutional Review Board of the Asan Medical Center (approval no. 2015-0736).
Postoperative MRI, including DW-MRI, was performed on every patient with or without a suspected stroke. Among the 556 patients, 8 (1.44%) were excluded because of a diagnosed brain infarction with neurological symptoms within 24 h after the operation, and so 548 patients were finally analyzed.
CEA was conducted in asymptomatic patients with >70% stenosis of the carotid artery confirmed by carotid duplex sonography or with ≥80% stenosis confirmed by computed tomography (CT) angiography or magnetic resonance (MR) angiography if the ultrasound investigations indicated 50–69% stenosis. In symptomatic patients, CEA was conducted in those with >50% stenosis of the ipsilateral carotid artery confirmed by carotid duplex sonography or with ≥70% stenosis confirmed by CT angiography or MR angiography if the ultrasound-detected stenosis was 50–69%. Symptoms related to carotid artery lesions included transient or persistent monocular visual loss, hemispheric transient ischemic attacks, nondisabling stroke, and ischemic stroke during the previous 6 months in the relevant carotid artery region.12 Regardless of the medical treatment applied, the indication for CEA was determined solely by the patient's symptoms and the region of carotid artery stenosis.
CEA was performed by three expert vascular surgeons who had each performed this procedure more than 30 times per year for more than 10 years. General or regional anesthesia was administered to all patients. All endarterectomies were open, with careful dissection performed of the bifurcation into internal carotid artery (ICA) and the external carotid artery. Some patients underwent shunt insertion from the common carotid artery to the ICA with a Javid shunt or Pruitt-Inahara shunt depending on the preference of the surgeon. In cases of general anesthesia, the shunt was inserted to reduce the cerebral oxygen saturation as measured by cerebral oximetry during the clamping time. In cases of regional anesthesia, the shunt was inserted if neurological symptoms such as mental change or dysarthria appeared during the clamping time. All patients were routinely administered an intravenous bolus of unfractionated heparin (80 units per kilogram body weight) before performing ICA cross-clamping. Bovine pericardial patches were applied in 513 (93.61%) of the 548 patients treated for ICA repair at our hospital. Additionally, closures were made using primary repair in 8 patients, an autogenous vein patch in 13 patients, and a polytetrafluoroethylene patch in 1 patient, while 13 patients underwent the eversion technique only. A Jackson-Pratt drain was placed on the platysma muscle layer when closing the wound in all patients. An antiplatelet agent had been applied in 332 (60.58%) patients preoperatively: 69 patients received clopidogrel (75 mg) only, 75 received acetylsalicylic acid (100 mg) only, and 188 patients received both antiplatelet agents. A statin was given to patients with a total fasting cholesterol level of higher than 200 mg/dL. Acetylsalicylic acid, clopidogrel, and a statin were administered immediately after CEA in all patients.
Patients were generally discharged on postoperative day 3 or 4, and a neurological examination was performed at the 1-month follow-up. If no complications were found, another follow-up was performed 6 months later and then annually thereafter.
Microinfarcts were diagnosed based on DW-MRI and clinical symptoms. MRI studies were performed on postoperative day 2. Average diffusion coefficient maps were calculated from the DW-MRI images. Based on information from another study,8 microinfarcts were defined as tiny focal acute lesions (volume less than 0.5 cm3) observed on DW-MRI scans; the corresponding average diffusion coefficient maps were constructed by an experienced radiologist (Fig. 1). No neurological symptoms were expected in any of the patients in whom brain DW-MRI was performed. In the present study, a delayed postoperative infarction was defined as a lesion larger than 0.5 cm3 in volume with accompanying neurological symptoms. Such lesions were located on the ipsilateral side of the CEA hemisphere and occurred later than postoperative day 2 (after the initial DW-MRI). Patients with stroke caused by cardioembolism or other determined etiology according to Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification13 were excluded based on the delayed postoperative infarction criteria.
Variables were compared between patients with and without microinfarcts. Continuous variables are presented as mean±standard-deviation values. Continuous and categorical variables were compared using Student's t-test and the chi-square test, respectively. Multiple logistic regression was used to analyze factors associated with microinfarcts by comparing the microinfarct-positive and microinfarct-negative groups. Univariate and multivariate analyses were used to identify risk factors associated with microinfarcts after CEA. Multivariate analysis was performed with factors that reached p<0.1 on univariate analysis.
The microinfarct-related, delayed postoperative infarction-free rates were estimated using the Kaplan-Meier method. Statistical analyses were performed using SPSS (Statistical Package for the Social Sciences, version 22, IBM, Chicago, IL, USA), and p<0.05 was used as the threshold for statistically significant differences.
Cerebral microinfarcts were found on DW-MRI in 76 patients (13.86%) after CEA. The patient characteristics and clinical data of the two groups are listed in Table 1. Univariate analysis indicated that the risk factors for microinfarcts (since their probability values reached p<0.1) were body mass index [odds ratio (OR)=1.07, 95% confidence interval (CI)=1.00–1.15, p=0.03], preoperative symptoms (OR=3.55, 95% CI=2.20–5.75, p<0.001), diabetes mellitus (OR=1.47, 95% CI=0.99–2.19, p=0.05), general anesthesia (OR=1.60, 95% CI=0.93–2.76, p=0.08), and shunt insertion (OR=1.65, 95% CI=0.95–2.84, p=0.07). Multivariate analysis performed using these factors (Table 2) showed that preoperative neurological symptoms were significantly related to the incidence of microinfarcts (OR=2.93, 95% CI=1.72–5.00, p<0.001). Among the operation-related variables, shunt insertion during CEA was the only significant risk factor for microinfarcts (OR=1.42, 95% CI=1.00–2.19, p<0.05) (Table 3).
Seven patients were diagnosed with delayed postoperative cerebral infarction among the cohort of 548 patients. An assessment of the association between postoperative microinfarcts and delayed postoperative infarction revealed that 2 of 76 (2.63%) microinfarct-positive patients and 5 of 472 (1.05%) microinfarct-negative patients were diagnosed with delayed infarction after CEA. The infarction vessel territory of patients with delayed postoperative infarction who were diagnosed with microinfarcts did not coincide exactly with the microinfarct vessel territory (Fig. 2) The infarction characteristics of all those with delayed postoperative infarction are presented in Table 4. The interval from CEA to postoperative infarction was 17.28–11.91 months (range=5–41 months). The presence of a postoperative microinfarct did not significantly increase the incidence of delayed postoperative infarction compared with those who underwent CEA without microinfarcts in the long term follow-up (p=0.211) (Fig. 3).
Based on our current findings and a review of the literature, we hypothesized that there are several possible causes of microinfarct. One is small particles originating from unstable plaques. A surgeon cannot avoid touching the plaque when dissecting the carotid artery, and this may result in plaque fragmentation that could lead to a microembolism. Small plaque-related particles such as lipids or blood clots will arise from the plaque, and they may cause microinfarcts. Furthermore, the rate of unstable carotid plaques is higher in symptomatic patients than in asymptomatic patients prior to surgery.14 The present study suggests that preoperative symptoms are relevant to microinfarcts due to the generation of such particles from unstable plaques.
The second possible cause of an microinfarct could be related to excessive manipulation of the carotid artery. Stork et al.9 reported risk factors for microembolism after CEA. That study revealed that being female and having left-side CEA are risk factors for microembolism. Those authors concluded that gentle manipulation was important. Female patients experience a higher rate of stroke after CEA because the vessel lumen is smaller than in the male ICA, which increases the technical difficulty of gentle manipulation during CEA. Stork et al.9 reported that the surgeons who participated in the study were all right-handed and hence found it difficult to perform the gentle manipulation for left-side CEA. Our present study is also consistent with the hypothesis that shunt insertion requires more handling than cases without shunt insertion. This means that selective shunt insertion during CEA would be preferable over obligatory shunt insertion. Several previous studies have aimed to identify clinical indications for shunting when performing carotid artery cross-clamping. Shin et al.15 suggested that a reduction in the patent segments in the contralateral hemisphere or an absence of both anterior and posterior communicating arteries were independent factors for shunting being required. However, to our knowledge, there is currently no definitive evidence to support the use of selective shunting.
The potential association between postoperative cerebral infarction and microinfarcts remains unclear. Some studies found that microinfarct lesions observed on DW-MRI after CAS did not resolve, instead progressing to cerebral infarctions on fluid-attenuated inversion recovery MRI images at 6 months after the procedure. These silent infarcts observed on MRI may contribute to an increased risk of dementia or a threefold increase in the risk of a subsequent stroke.11161718 However, Wolf et al.19 found no correlation between microembolism and cerebral infarction and no increase in the infarction rate in the microembolism group during a long-term follow-up. Levi et al.20 reported that microembolisms are associated with an increased risk of stroke and new ischemic lesions on MRI after CEA, and they suggested that a microembolism results in failure of the microcirculation due to frequent microembolic occlusion. However, that study was limited to the perioperative outcomes only, and so to assess the relationship between microembolism and stroke they assumed that this obstacle in microcirculation occurs only in the presence of frequent microembolic occlusion (in excess of 50 microembolic signals per hour, as measured using transcranial Doppler). In the present study we also found no association between microinfarcts and delayed postoperative infarction. Moreover, patients with delayed postoperative infarction who had been diagnosed with microinfarcts previously had no corresponding infarction lesion or microinfarct lesion.
This study was subject to some noteworthy limitations. First, we included only a small cohort of patients with delayed postoperative infarction (n=7). The inclusion of only seven cases (1.27% of the total cohort) restricted the ability to identify any statistically significant relationship between microinfarcts and postoperative infarctions. Second, the origin of postoperative microinfarcts could not be distinguished from the cardiac or intracranial vessel origin. Third, there was a lack of baseline information for pre-existing microinfarcts for all patients. Fourth, acute hemodynamic changes may contribute to the occurrence of microembolisms during CEA, but unfortunately we were unable to collect the relevant information on blood pressure when reviewing the data.
There are many risk factors for microinfarcts after CEA, including preoperative symptoms and the intraoperative insertion of a shunt. Therefore, when preparing symptomatic patients for CEA, selective shunt insertion should be considered and plaque stability should be evaluated. We found no association between microinfarcts after CEA and delayed postoperative infarction.
Figures and Tables
Fig. 1
DW-MRI images of an 80-year-old female patient who underwent imaging 2 days after CEA. Multiple tiny foci that exhibit restricted diffusion (white arrows) represent microinfarct lesions. CEA: carotid endarterectomy, DW-MRI: diffusion-weighted magnetic resouance imaging.
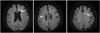
Fig. 2
DW-MRI images obtained on postoperative day 2 showing microinfarcts in the middle cerebral artery and posterior cerebral artery border zone (white arrows), and DW-MRI images obtained 14 months postoperatively showing infarction in the middle cerebral artery territory (yellow arrow). DW-MRI: diffusion-weighted magnetic resonance imaging.
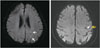
Table 1
Baseline characteristics of the study patients

Table 2
Results of univariate analysis of risk factors influencing microembolization after CEA
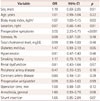
Table 3
Results of multivariate analysis of risk factors influencing microembolization after CEA
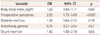
Table 4
Infarction characteristics of the patients with delayed postoperative cerebral infarction

References
1. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991; 325:445–453.
2. Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004; 363:1491–1502.

3. Rothwell PM, Gutnikov SA, Warlow CP. European Carotid Surgery Trialist's Collaboration. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke. 2003; 34:514–523.

4. Gupta N, Corriere MA, Dodson TF, Chaikof EL, Beaulieu RJ, Reeves JG, et al. The incidence of microemboli to the brain is less with endarterectomy than with percutaneous revascularization with distal filters or flow reversal. J Vasc Surg. 2011; 53:316–322.

5. Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003; 60:1394–1399.

6. Yamashita H, Fujikawa T, Takami H, Yanai I, Okamoto Y, Morinobu S, et al. Long-term prognosis of patients with major depression and silent cerebral infarction. Neuropsychobiology. 2010; 62:177–181.

7. Wilson TJ, Pandey AS, Stetler WR Jr, Davis MC, Giles DA, Chaudhary N, et al. Dual antiplatelet therapy plus postoperative heparin and dextran is safe and effective for reducing risk of embolic stroke during aneurysm coiling. Acta Neurochir (Wien). 2014; 156:855–859.

8. Kuliha M, Roubec M, Procházka V, Jonszta T, Hrbáč T, Havelka J, et al. Randomized clinical trial comparing neurological outcomes after carotid endarterectomy or stenting. Br J Surg. 2015; 102:194–201.

9. Stork JL, Levi CR, Chambers BR, Abbott AL, Donnan GA. Possible determinants of early microembolism after carotid endarterectomy. Stroke. 2002; 33:2082–2085.

10. Levi CR, Roberts AK, Fell G, Hoare MC, Royle JP, Chan A, et al. Transcranial Doppler microembolus detection in the identification of patients at high risk of perioperative stroke. Eur J Vasc Endovasc Surg. 1997; 14:170–176.

11. King A, Markus HS. Doppler embolic signals in cerebrovascular disease and prediction of stroke risk: a systematic review and meta-analysis. Stroke. 2009; 40:3711–3717.

12. Mantese VA, Timaran CH, Chiu D, Begg RJ, Brott TG. CREST Investigators. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): stenting versus carotid endarterectomy for carotid disease. Stroke. 2010; 41:10 Suppl. S31–S34.
13. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.

14. Gaunt ME, Brown L, Hartshorne T, Bell PR, Naylor AR. Unstable carotid plaques: preoperative identification and association with intraoperative embolisation detected by transcranial Doppler. Eur J Vasc Endovasc Surg. 1996; 11:78–82.

15. Shin S, Kwon TW, Cho YP, Shin JH, Yi A, Kim H, et al. Preoperative magnetic resonance angiography as a predictive test for cerebral ischemia during carotid endarterectomy. World J Surg. 2013; 37:663–670.

16. Palombo G, Faraglia V, Stella N, Giugni E, Bozzao A, Taurino M. Late evaluation of silent cerebral ischemia detected by diffusion-weighted MR imaging after filter-protected carotid artery stenting. AJNR Am J Neuroradiol. 2008; 29:1340–1343.

17. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003; 348:1215–1222.

18. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Rotterdam Scan Study. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003; 34:1126–1129.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download