This article has been
cited by other articles in ScienceCentral.
Abstract
Background and Purpose
Responses to repetitive nerve stimulation (RNS) in patients with muscle-specific tyrosine kinase (MuSK) antibody (Ab)-positive myasthenia gravis (MG) vary depending on the muscles tested. We analyzed the RNS responses of limb and facial muscles in MuSK-Ab-positive and acetylcholine receptor (AChR)-Ab-negative MG (MuSK MG) and MuSK-Ab-negative and AChR-Ab-negative [double-seronegative (DSN)] MG patients.
Methods
We retrospectively compared RNS responses between 45 MuSK MG and 29 DSN MG. RNS was applied to the abductor digiti minimi, flexor carpi ulnaris, trapezius, orbicularis oculi, and nasalis muscles.
Results
Abnormal RNS responses in limb muscles were observed in 22.2 and 58.6% of MuSK MG and DSN MG patients, respectively, with abnormal facial responses observed in 77.8 and 65.5%, and abnormal responses observed in any of the five muscles in 86.7 and 72.4%. Abnormal RNS responses in the abductor digiti minimi or flexor carpi ulnaris were less frequent in MuSK MG (8.9 and 15.6%, respectively) than in DSN MG (37.9 and 55.2%), whereas the findings for other muscles were not significantly different between the groups. Abnormal facial responses but normal limb responses were independently associated with MuSK MG (odds ratio=5.224, 95% confidence interval=1.300–20.990).
Conclusions
Abnormal RNS responses primarily in facial muscles without involvement of limb muscles were more pronounced in MuSK MG than in DSN MG. RNS of both facial and limb muscles in AChR-Ab-negative MG can increase the test sensitivity and aid in early suspicion of MuSK MG.
Go to :

Keywords: MuSK, muscle-specific tyrosine kinase, myasthenia gravis, repetitive nerve stimulation, acetylcholine receptor
INTRODUCTION
Myasthenia gravis (MG) is a relatively rare autoimmune disorder characterized by fatigable weakness of voluntary muscles.
12 Since impaired neuromuscular transmission is the main pathomechanism of MG, identifying this is essential in an MG diagnosis. Therefore, electrodiagnostic studies including repetitive nerve stimulation (RNS) testing are important diagnostic tools, especially in patients without antibodies (Abs) to the acetylcholine receptor (AChR).
Typically 40–70% of generalized MG patients without AChR Abs have Abs to muscle-specific tyrosine kinase (MuSK).
3456 The few studies in which RNS was performed in patients with MuSK-Ab-positive MG produced wide ranges for the proportion of patients with abnormal responses (12% to 86%).
78910 These previous studies were heterogeneous with respect to the nerve–muscle sets studied in the RNS: whereas some studies performed RNS only in limb muscles,
37 others performed RNS in both limb and facial muscles.
5101112 Also, most previous studies had small populations.
569
The aim of the present study was to determine the difference in sensitivity of RNS according to the tested muscles and reveal any distinctive RNS features of MuSK-Ab-positive MG patients among AChR-Ab-negative MG patients. We analyzed the RNS responses of limb and facial muscles in MuSK-Ab-positive and AChR-Ab-negative MG (MuSK MG) patients and compared the results with those of MuSK-Ab-negative and AChR-Ab-negative [double-seronegative (DSN)] MG patients.
Go to :

METHODS
Subjects
We retrospectively reviewed medical records of MG patients between January 1992 and December 2015 in Severance Hospital. The diagnosis of MG was based on clinical symptoms and signs of muscle fatigue, decremental responses to low-frequency RNS, serum levels of AChR and MuSK Abs, and improvement of muscle fatigue after the intramuscular injection of neostigmine. The following inclusion criteria were used: 1) completed AChR Ab and MuSK Ab assay, 2) negative AChR Ab results, and 3) RNS performed in all of five muscles (abductor digiti minimi, flexor carpi ulnaris, nasalis, orbicularis oculi, and trapezius). In total, 81 AChR-Ab-negative MG patients met the inclusion criteria, of which 8 with pure ocular MG at the time of RNS and 1 with concomitant diagnosis of amyotrophic lateral sclerosis were excluded. All of the pure ocular cases were DSN MG patients. Finally, data from 45 MuSK MG patients (i.e., negative for AChR Ab and positive for MuSK Ab) and 29 DSN MG patients (i.e., negative for both AChR and MuSK Abs) were analyzed. The University of Yonsei Institutional Review Board approved this research (Approval No. 4-2016-0775).
Diagnostic testing
RNS was performed using the method of Oh et al.
13 in five muscles: the abductor digiti minimi and flexor carpi ulnaris after ulnar nerve stimulation, the orbicular oculi and nasalis after facial nerve stimulation, and the trapezius after spinal accessory nerve stimulation. Nerve stimulation was performed by delivering supramaximal stimulation for 0.2 ms. The compound muscle action potential (CMAP) was recorded in each tested muscle at baseline and immediately after 30 seconds of muscle exercise. Low-frequency RNS was performed at 1, 3, and 5 minutes after exercise, with five 3-Hz stimulations delivered at 3 minutes after exercise. The responses to RNS at 3 minutes after exercise were analyzed in this study. The ratio between the first CMAP and the lowest CMAP among the first five waveforms was calculated. A decrement in CMAP of ≥10% was considered abnormal. In those patients taking anticholinesterase agents, RNS was conducted at least 12 hours after the last dose. RNS was conducted using the Neuroscreen System (Toennies, Bavaria, Germany) or the Schwarzer topas EMG system (Natus, Bavaria, Germany). The AChR Ab was analyzed using a commercially available assay (anti-AChR-binding Ab, Seoul Clinical Laboratories, Seoul, Korea), with the result considered negative when the Ab level was ≤0.2 nmol/L. An anti-MuSK Ab assay was also conducted using a commercially available assay (anti-MuSK Ab, Athena Diagnostics, Worcester, MA, USA), with the results categorized into negative (<10 titer units), borderline (≥10 titer units and <20 titer units), or positive (≥20 titer units); those patients categorized as positive were included in the MuSK MG group.
Data collection
Clinical features including age, sex, age at symptom onset, age at initial RNS, symptoms at disease onset, Myasthenia Gravis Foundation of America (MGFA) clinical classification, quantitative myasthenia gravis (QMG) score, state of immunosuppressive treatment, and presence of MG crisis were collected by reviewing medical records. RNS results at the time of initial visit to our clinic were also recorded based on electrodiagnostic reports.
Statistical analysis
Fisher's exact test or the chi-square test was used to compare categorical variables, and the t-test or Mann-Whitney test was used to compare continuous variables. The associations between clinical and electrodiagnostic variables and MuSK MG were evaluated using multiple logistic regression analyses. Covariates with p value <0.1 in the univariate analyses were included in multivariate analyses. Because of the relatively small sample, the MGFA classification was included as a representative of clinical features in the multivariate analysis. Patients who were assigned a MGFA classification of “b” were regarded as those with bulbar predominance. All statistical analyses were two-tailed, and a p value <0.05 was considered to be statistically significant. Statistical analyses were performed using R software (version 3.2.2, R Foundation, Vienna, Austria).
Go to :

RESULTS
Clinical characteristics
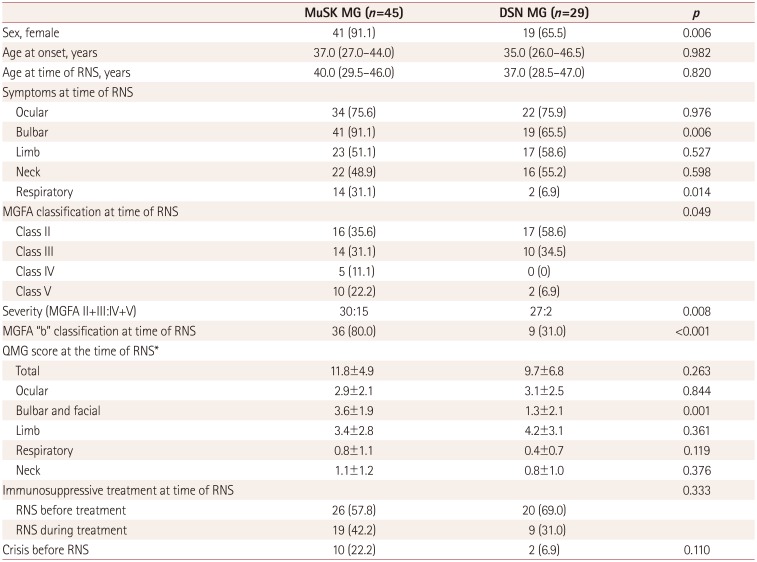
The clinical features in the MuSK MG and DSN MG groups are listed in
Table 1. The proportion of females was greater in the MuSK MG group (91.1%) than in the DSN MG group (65.5%,
p=0.006). Bulbar and respiratory symptoms were more frequent in the MuSK MG group (91.1 and 31.1%) than in the DSN MG group (65.5 and 6.9%,
p=0.006 and 0.014, respectively). A MGFA classification of IV or V was more frequent in the MuSK MG group (33.3%) than in the DSN MG group (6.9%,
p=0.008). Among the subgroup of patients whose QMG scores were recorded, the total QMG score did not differ between the two groups. However, the QMG score for bulbar and facial weakness was higher in MuSK MG (3.6±1.9, mean±standard deviation) than in DSN MG (1.3±2.1,
p=0.001). The state of immunosuppressive treatment or the presence of an MG crisis before the initial RNS did not differ between the two groups.
Table 1
Clinical features of MuSK-Ab-positive and AChR-Ab-negative MG (MuSK MG) patients and MuSK-Ab-negative and AChR-Ab-negative (DSN) MG patients at the time of an initial RNS test
|
MuSK MG (n=45) |
DSN MG (n=29) |
p
|
|
Sex, female |
41 (91.1) |
19 (65.5) |
0.006 |
|
Age at onset, years |
37.0 (27.0–44.0) |
35.0 (26.0–46.5) |
0.982 |
|
Age at time of RNS, years |
40.0 (29.5–46.0) |
37.0 (28.5–47.0) |
0.820 |
|
Symptoms at time of RNS |
|
|
|
|
Ocular |
34 (75.6) |
22 (75.9) |
0.976 |
|
Bulbar |
41 (91.1) |
19 (65.5) |
0.006 |
|
Limb |
23 (51.1) |
17 (58.6) |
0.527 |
|
Neck |
22 (48.9) |
16 (55.2) |
0.598 |
|
Respiratory |
14 (31.1) |
2 (6.9) |
0.014 |
|
MGFA classification at time of RNS |
|
|
0.049 |
|
Class II |
16 (35.6) |
17 (58.6) |
|
|
Class III |
14 (31.1) |
10 (34.5) |
|
|
Class IV |
5 (11.1) |
0 (0) |
|
|
Class V |
10 (22.2) |
2 (6.9) |
|
|
Severity (MGFA II+III:IV+V) |
30:15 |
27:2 |
0.008 |
|
MGFA “b” classification at time of RNS |
36 (80.0) |
9 (31.0) |
<0.001 |
|
QMG score at the time of RNS* |
|
|
|
|
Total |
11.8±4.9 |
9.7±6.8 |
0.263 |
|
Ocular |
2.9±2.1 |
3.1±2.5 |
0.844 |
|
Bulbar and facial |
3.6±1.9 |
1.3±2.1 |
0.001 |
|
Limb |
3.4±2.8 |
4.2±3.1 |
0.361 |
|
Respiratory |
0.8±1.1 |
0.4±0.7 |
0.119 |
|
Neck |
1.1±1.2 |
0.8±1.0 |
0.376 |
|
Immunosuppressive treatment at time of RNS |
|
|
0.333 |
|
RNS before treatment |
26 (57.8) |
20 (69.0) |
|
|
RNS during treatment |
19 (42.2) |
9 (31.0) |
|
|
Crisis before RNS |
10 (22.2) |
2 (6.9) |
0.110 |

Comparison of repetitive nerve stimulation between groups
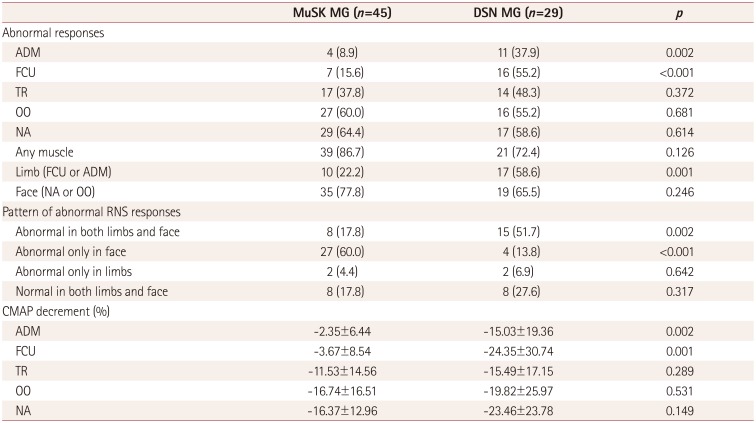
The RNS results for the MuSK MG and DSN MG groups are presented in
Table 2. Abnormal RNS responses in the abductor digiti minimi or flexor carpi ulnaris occurred less frequently in the MuSK MG group (8.9 and 15.6%) than in the DSN MG group (37.9 and 55.2%,
p=0.002 and
p<0.001, respectively). By contrast, the proportions of abnormal RNS responses in the trapezius, orbicularis oculi, and nasalis muscles were not significantly different across the groups. The magnitude of the CMAP decrement showed similar patterns. The CMAP decrement in the abductor digiti minimi or flexor carpi ulnaris muscles was significantly greater in the DSN MG group (-15.03 and -24.35%, respectively) than in the MuSK MG group (-2.35 and -3.67%,
p=0.002 and 0.001, respectively). In contrast, the CMAP decrement in the orbicularis oculi, nasalis, and trapezius muscles did not differ between the two groups.
Table 2
Initial RNS results for MuSK MG and DSN MG patients
|
MuSK MG (n=45) |
DSN MG (n=29) |
p
|
|
Abnormal responses |
|
|
|
|
ADM |
4 (8.9) |
11 (37.9) |
0.002 |
|
FCU |
7 (15.6) |
16 (55.2) |
<0.001 |
|
TR |
17 (37.8) |
14 (48.3) |
0.372 |
|
OO |
27 (60.0) |
16 (55.2) |
0.681 |
|
NA |
29 (64.4) |
17 (58.6) |
0.614 |
|
Any muscle |
39 (86.7) |
21 (72.4) |
0.126 |
|
Limb (FCU or ADM) |
10 (22.2) |
17 (58.6) |
0.001 |
|
Face (NA or OO) |
35 (77.8) |
19 (65.5) |
0.246 |
|
Pattern of abnormal RNS responses |
|
|
|
|
Abnormal in both limbs and face |
8 (17.8) |
15 (51.7) |
0.002 |
|
Abnormal only in face |
27 (60.0) |
4 (13.8) |
<0.001 |
|
Abnormal only in limbs |
2 (4.4) |
2 (6.9) |
0.642 |
|
Normal in both limbs and face |
8 (17.8) |
8 (27.6) |
0.317 |
|
CMAP decrement (%) |
|
|
|
|
ADM |
−2.35±6.44 |
−15.03±19.36 |
0.002 |
|
FCU |
−3.67±8.54 |
−24.35±30.74 |
0.001 |
|
TR |
−11.53±14.56 |
−15.49±17.15 |
0.289 |
|
OO |
−16.74±16.51 |
−19.82±25.97 |
0.531 |
|
NA |
−16.37±12.96 |
−23.46±23.78 |
0.149 |

The anatomical distribution of abnormal RNS responses differed between MuSK MG and DSN MG. An RNS pattern that was abnormal in facial muscles (abnormal in either the orbicularis oculi or nasalis) and normal in limb muscles (normal in both the abductor digiti minimi and flexor carpi ulnaris) occurred significantly more frequently in the MuSK MG group (60%) than in the DSN MG group (13.8%, p<0.001). A pattern that was abnormal in both facial and limb muscles was more frequent in DSN MG (51.7%) than in MuSK MG (17.8%, p=0.002).
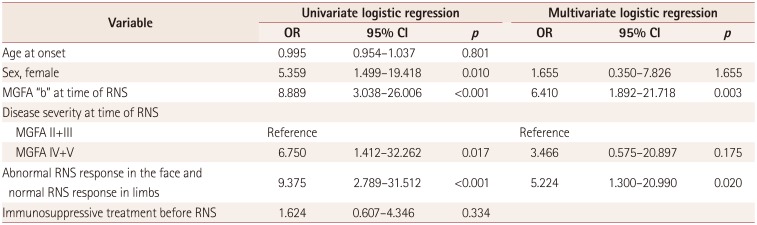
Multivariate analysis
Multiple logistic regression analysis was performed to assess clinical and electrodiagnostic factors associated with MuSK MG. In univariate analysis, female sex, bulbar MGFA classification, severity of disease, and RNS patterns that were abnormal in the face but normal in limbs were related to MuSK MG. In multivariate analysis, bulbar MGFA classification [odds ratio (OR)=6.410, 95% confidence interval (CI)=1.892–21.718] and RNS patterns that were abnormal in the face but normal in limbs (OR=5.224, 95% CI=1.300–20.990) were found to be independently associated with MuSK MG (
Table 3).
Table 3
Results of univariate and multivariate analyses evaluating the clinical and electrodiagnostic factors associated with MuSK MG compared to DSN MG
|
Variable |
Univariate logistic regression |
Multivariate logistic regression |
|
OR |
95% CI |
p
|
OR |
95% CI |
p
|
|
Age at onset |
0.995 |
0.954–1.037 |
0.801 |
|
|
|
|
Sex, female |
5.359 |
1.499–19.418 |
0.010 |
1.655 |
0.350–7.826 |
1.655 |
|
MGFA “b” at time of RNS |
8.889 |
3.038–26.006 |
<0.001 |
6.410 |
1.892–21.718 |
0.003 |
|
Disease severity at time of RNS |
|
|
|
|
|
|
|
MGFA II+III |
Reference |
|
|
Reference |
|
|
|
MGFA IV+V |
6.750 |
1.412–32.262 |
0.017 |
3.466 |
0.575–20.897 |
0.175 |
|
Abnormal RNS response in the face and normal RNS response in limbs |
9.375 |
2.789–31.512 |
<0.001 |
5.224 |
1.300–20.990 |
0.020 |
|
Immunosuppressive treatment before RNS |
1.624 |
0.607–4.346 |
0.334 |
|
|
|

Change in sensitivity of repetitive nerve stimulation
Overall, abnormal responses in any of the five muscles were observed in 86.7% of MuSK MG patients and 72.4% of DSN MG patients (
Fig. 1). Abnormal responses in limb muscles were observed in 22.2% of MuSK MG patients and 58.6% of DSN MG patients. When the trapezius muscle was additionally evaluated, the proportion of abnormal responses increased to 48.9% in the MuSK MG group, whereas the sensitivity did not change in the DSN MG group. When considering both the limb and facial muscles, the proportion of patients with abnormal RNS responses increased to 82.2% in the MuSK MG group and 72.4% in the DSN MG group.
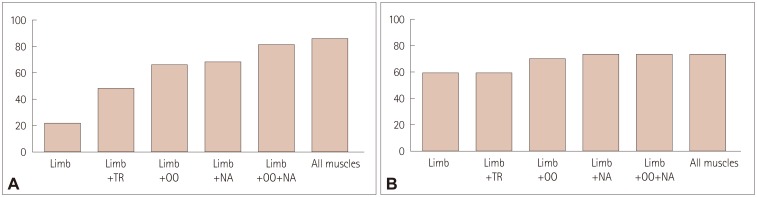 | Fig. 1Abnormal rate of repetitive nerve stimulation responses depending on the site of examination in (A) muscle-specific tyrosine kinase-antibody-positive myasthenia gravis group and (B) double-seronegative myasthenia gravis group. NA: nasalis, OO: orbicularis oculi, TR: trapezius.
|
Go to :

DISCUSSION
Abnormal RNS responses of limb muscles occurred less frequently in MuSK MG patients than in DSN MG patients. A pattern of abnormal facial muscle responses but normal limb muscle responses was more frequent in MuSK MG patients than in DSN MG patients. Whereas the proportion of MuSK MG patients with abnormal responses was 22.2% when considering only limb muscles, this increased to 82.2% when the orbicularis oculi and nasalis muscles were also evaluated. By contrast, the additional evaluation of facial muscles in DSN MG patients increased the sensitivity by only 13.8%. These findings suggest that abnormal RNS responses primarily in facial muscles without involvement of distal limb muscles are more pronounced in MuSK MG than in DSN MG, and that performing RNS in both the face and limbs can increase the test sensitivity.
Our findings are consistent with those of previous studies. Some of the previous studies found a low sensitivity of RNS in MuSK-Ab-positive MG patients, but they only performed RNS in hand or shoulder muscles and not in facial muscles.
67 Consistent with this, we found abnormal RNS responses in limb muscles in only 22.2% of MuSK MG patients. Other studies that performed RNS in both limb and facial muscles found abnormal responses to be more common in facial muscles (75–80%) than in limb muscles (25–36%).
81114 In the present study, the sensitivities of RNS in the facial and limb muscles were 77.8 and 22.2%, respectively. In addition, the RNS responses tended to be abnormal only in the face, and rarely in both the face and limbs, which is consistent with a previous study suggesting focal muscle involvement in MuSK-Ab-positive MG.
10
The proportion of abnormal RNS responses in the present DSN MG patients is also consistent with previous studies. Abnormal RNS responses were reportedly observed in 30–74% of subjects in facial muscles and in 36–78% of subjects in limb muscles.
391112 Similarly, in the present study, the RNS responses of facial and limb muscles were abnormal in 65.5 and 58.6% of DSN MG patients, respectively.
The strengths of the present study include its relatively large population and the similarity of the methods used to perform RNS across patients. By contrast, previous studies have included relatively small populations or recruited patients from different institutions, and did not perform RNS in the same muscles.
8914 Also, in the present study we were able to calculate the sensitivity of RNS in each tested muscle and demonstrate how the sensitivity changed as the number of muscles tested increased. This revealed that additionally evaluating the orbicularis oculi and nasalis muscles increased the overall sensitivity in MuSK MG by 60%.
Early suspicion of MuSK-Ab-positive MG is important since this condition is often associated with rapid disease progression, frequent respiratory crisis, poor response to cholinesterase inhibitor, and early requirement of immunosuppressive treatment.
1516 Because bulbar and facial muscle weakness is prominent in MuSK-Ab-positive MG, this condition can be suspected based on clinical characteristics. However, a differential diagnosis is difficult based solely on clinical data. In the present study, abnormal RNS responses primarily in facial muscles were shown to be independently associated with MuSK MG after adjusting for sex, disease severity, and clinical presentation. RNS is usually performed when MG is initially suspected, with the results being immediately available to physicians after the test. Thus, identification of the typical RNS pattern of MuSK MG can contribute to early diagnoses.
In conclusion, conducting RNS in multiple muscles including those of the face and limbs in AChR-Ab-negative MG patients increases the diagnostic sensitivity and may be helpful in differentiating MuSK MG from DSN MG.
Go to :

Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No. NRF-2016R1C1B1010120).
Go to :

Notes
Go to :

References
1. Gilhus NE. Myasthenia gravis. N Engl J Med. 2016; 375:2570–2581. PMID:
28029925.

2. Lee HS, Lee HS, Shin HY, Choi YC, Kim SM. The epidemiology of myasthenia gravis in Korea. Yonsei Med J. 2016; 57:419–425. PMID:
26847295.

3. Evoli A, Tonali PA, Padua L, Monaco ML, Scuderi F, Batocchi AP, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain. 2003; 126(Pt 10):2304–2311. PMID:
12821509.

4. Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001; 7:365–368. PMID:
11231638.

5. Lavrnic D, Losen M, Vujic A, De Baets M, Hajdukovic LJ, Stojanovic V, et al. The features of myasthenia gravis with autoantibodies to MuSK. J Neurol Neurosurg Psychiatry. 2005; 76:1099–1102. PMID:
16024887.

6. Sanders DB, El-Salem K, Massey JM, McConville J, Vincent A. Clinical aspects of MuSK antibody positive seronegative MG. Neurology. 2003; 60:1978–1980. PMID:
12821744.

7. Padua L, Tonali P, Aprile I, Caliandro P, Bartoccioni E, Evoli A. Seronegative myasthenia gravis: comparison of neurophysiological picture in MuSK+ and MuSK- patients. Eur J Neurol. 2006; 13:273–276. PMID:
16618345.

8. Pasnoor M, Wolfe GI, Nations S, Trivedi J, Barohn RJ, Herbelin L, et al. Clinical findings in MuSK-antibody positive myasthenia gravis: a U.S. experience. Muscle Nerve. 2010; 41:370–374. PMID:
19882635.

9. Oh SJ, Hatanaka Y, Hemmi S, Young AM, Scheufele ML, Nations SP, et al. Repetitive nerve stimulation of facial muscles in MuSK antibody-positive myasthenia gravis. Muscle Nerve. 2006; 33:500–504. PMID:
16392120.

10. Nikolic A, Basta I, Stojanovic VR, Stevic Z, Lavrnic D. Electrophysiological profile of the patients with MuSK positive myasthenia gravis. Neurol Res. 2014; 36:945–949. PMID:
24825477.

11. Oh SJ. Muscle-specific receptor tyrosine kinase antibody positive myasthenia gravis current status. J Clin Neurol. 2009; 5:53–64. PMID:
19587811.

12. Wolfe GI, Oh SJ. Clinical phenotype of muscle-specific tyrosine kinase-antibody-positive myasthenia gravis. Ann N Y Acad Sci. 2008; 1132:71–75. PMID:
18567855.

13. Oh SJ, Eslami N, Nishihira T, Sarala PK, Kuba T, Elmore RS, et al. Electrophysiological and clinical correlation in myasthenia gravis. Ann Neurol. 1982; 12:348–354. PMID:
7149660.

14. Stickler DE, Massey JM, Sanders DB. MuSK-antibody positive myasthenia gravis: clinical and electrodiagnostic patterns. Clin Neurophysiol. 2005; 116:2065–2068. PMID:
16043398.

15. Evoli A, Bianchi MR, Riso R, Minicuci GM, Batocchi AP, Servidei S, et al. Response to therapy in myasthenia gravis with anti-MuSK antibodies. Ann N Y Acad Sci. 2008; 1132:76–83. PMID:
18567856.

16. Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve. 2011; 44:36–40. PMID:
21674519.

Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download