Abstract
Background and Purpose
Few studies of dementia in Parkinson's disease (PD) have had long-term follow-ups. Moreover, information on the duration from the onset to the development of dementia in patients with PD is lacking. The aim of this study was to determine the median dementia-free survival time from the onset of PD to the development of dementia.
Methods
In total, 1,193 Korean patients with PD were recruited and assessed at regular intervals of 3–6 months. We interviewed the patients and other informants to identify impairments in the activities of daily living. The Hoehn and Yahr stage and scores on the Unified Parkinson's Disease Rating Scale and Mini Mental State Examination were evaluated annually. We used Kaplan-Meier survival analysis to estimate the cumulative proportion of dementia-free patients over time. Risk factors predicting dementia were also evaluated using Cox proportional-hazards regression models.
Results
The median dementia-free survival time in the Korean PD population was 19.9 years. Among the 119 patients who subsequently developed dementia, the mean duration from the onset of PD to the development of dementia was 10.6 years. A multivariate analysis identified age at onset and education period as the significant predictors of dementia.
Dementia has been recognized as one of the most important nonmotor symptoms of Parkinson's disease (PD) since it crucially affects both the quality of life and burden of care.1 Previous studies of the prevalence of dementia in PD have produced varying results that are usually influenced by the methodology employed. For example, a previous review using strict methodological criteria found that the prevalence of dementia in PD was 31.3%,2 and this finding was supported by studies suggesting that the rate of dementia in PD ranges between 22% (in the Rotterdam Study) and 44% (in people with PD older than 60 years).34 However, these studies underestimated the true prevalence of dementia, since the mortality rate tends to be higher among patients with dementia than among patients without dementia with PD.5 In addition, reports on the point prevalence do not provide information on the cumulative proportion of patients with PD who develop dementia. A recent study conducted in Sydney with a 20-year follow-up found that 83% of the surviving 30 PD patients had dementia, and 75% of all patients had developed dementia prior to death.6 However, these studies also do not provide information on the duration from the onset of PD to the development of dementia.
A report on a survival analysis including the period from the onset of disease7 did not specify how the time when dementia developed in the patients who already developed dementia before the baseline was determined. Given that the exact timing of events is crucial for survival analysis, the prospective observation of patients is mandatory. Only regular neuropsychological testing over long intervals can detect the exact timing of when dementia first develops and provide information on impairments in the activities of daily living (ADL). Given that such impairments are critical in the diagnosis of dementia, and are judged by a clinician, serial assessment based on close examinations and interviews of patients and other informants is crucial for the diagnosis of dementia. Studies employing serial assessment of the exact timing of when dementia first develops and providing information on the duration from the disease onset to dementia development in a population of patients with PD have not been reported previously.
The purpose of this study was to provide information on the median dementia-free survival time from PD onset to the development of dementia in a PD population through serial assessment using survival analysis. The risk factors predicting dementia in PD were also investigated.
Subjects were recruited from the Dong-A Alzheimer and Parkinson Registry. A total of 2,459 PD patients visited the Parkinson's Disease Center of Dong-A University for the first time between January 2001 (when the disease code for PD and dementia in PD was established in Korea) and August 2014. The disease duration was less than 3 years in 1,117 of the patients, and they were not included in this study in order to exclude patients with atypical or secondary parkinsonism, thereby increasing the diagnostic accuracy. Seventeen patients whose exact age was unknown when dementia developed were also excluded: 12 showed dementia when they first visited the clinic and 5 showed dementia but did not visit our clinic for more than 1 year during the follow-up period. Other exclusion criteria were 1) being illiterate (n=73), 2) receiving deep brain stimulation (n=46), 3) having major depression (n=2), 4) having a major stroke affecting cognition (n=2), 5) having life-threatening cancer (n=5), and 6) taking anticholinergics on a regular basis (n=4). A total of 1,193 Korean patients finally entered the study. The clinical diagnosis of PD was based on criteria of the United Kingdom Parkinson's Disease Society Brain Bank.8 All subjects gave their informed consent, and the local Institutional Review Board approved this study.
All patients were evaluated in a neurological examination and interviewed with semi-constructed questionnaires for the history of illness, medication, and the responsiveness to medication. The Hoehn and Yahr (HY) stage9 and scores for Part 1 and items 20 (tremor at rest), 22 (rigidity), 23 (finger taps), and 26 (leg agility) of Part 3 of the Unified Parkinson's Disease Rating Scale (UPDRS)10 and for the Mini Mental State Examination (MMSE)11 were evaluated as a baseline measurement and then repeated once a year. A depression score was obtained using the Geriatric Depression Scale.12 Patients who were suspected of having dementia during the follow-up period received a comprehensive neuropsychological test using the Seoul Neuropsychological Screening Battery (SNSB)13 or the Clinical Dementia Rating (CDR).14 Additionally, the family members or caregivers of these patients were interviewed to confirm that the ADL impairment was caused by cognitive decline and not by motor disabilities. For those who were lost to follow-up, the latest data were used for analysis and the reasons for the loss to follow-up were not investigated.
The diagnosis of dementia in PD was made based on criteria from the Fourth Edition of the Diagnostic and Statistical Manual of Mental Disorders. The patients had to exhibit impairment in multiple domains including memory and executive function in the SNSB. If an SNSB rating was not available, a diagnosis of dementia was based on a CDR ≥1, with evidence from family members that the patient's gradual cognitive decline was sufficient to impair the ADL. Additionally, the patient had to fulfill at least one of the following criteria: 1) MMSE score of less than 24 and 2 standard deviations (SDs) below the mean of age- and education-matched controls and 2) score of 2 or more on the Intellectual Impairment item of UPDRS Part 1.
The cumulative proportion of patients becoming demented with the passage of time was estimated using Kaplan-Meier survival analysis. The event of interest was the occurrence of dementia, and the proportion of patients surviving without dementia at each time point was the proportion of patients who had not become demented. The time to an event was surveyed at the end of the study or follow-up if the event had not occurred by that time. To investigate which baseline variables were associated with subsequent dementia, univariate analyses of the association of each variable with dementia were followed by multivariate analysis using variables with a p value of 0.20 or lower in univariate analysis, and several clinically significant variables were also included. For the univariate analyses, each continuous variable was dichotomized at its median value. The categorical variables examined were sex and presence or absence of chronic disease (hypertension or diabetes mellitus). Cox proportional-hazards regression models were used for both the univariate and multivariate analyses. The backward method of variable selection was used for multivariate analysis. Additionally, the log-rank test was used to compare survival curves according to age at onset. All analyses was carried out using the SAS computer package (version 9.2, SAS Institute, Cary, NC, USA).
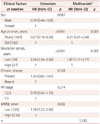
In total, 1,193 patients with PD were recruited, of which 523 (43.8%) were lost to follow-up. The age at disease onset of the 1,193 patients was 60.1±10.0 years (mean±SD) and the age when they first visited our clinic (i.e., the age at entry into the study) was 64.3±9.8 years (Table 1). The patients had a disease duration of 4.2±3.7 years before visiting our clinic (previsit disease duration), and the follow-up lasted 3.9±3.3 years. Therefore, the mean investigation period (previsit disease duration plus follow-up) was 8.1 years; it was less than 5 years in 171 patients (14.3%), 5–10 years in 755 (63.3%), 11–20 years in 260 (21.8%), and more than 21 years in 7 (0.6%). Dementia developed in 119 patients during follow-up, with a duration between the onset of PD to the development of dementia of 10.6±4.5 years. Their mean age at the time of diagnosis of dementia was 73.7 years. There was 1 patient (0.8%) aged 50–59 years at the time when dementia was diagnosed, 16 (13.5%) aged 60–69 years, 64 (53.8%) aged 70–79 years, and 38 (31.9%) older than 80 years. The education period, MMSE score, and HY stage at baseline were 7.15±5.11 years, 24.13±6.31, and 2.31±0.71, respectively. Some data of UPDRS scores, levodopa dose, and depression scores were missing, and so these variables were not included in the risk factors for dementia. Seventeen patients whose exact age was unknown when dementia developed showed no differences in clinical characteristics known to be risk factors for the development of dementia.
A total of 119 of the 1,193 patients met the criteria for dementia. The dementia-free survival time was calculated as the duration from the onset of PD to the development of dementia. The results of the Kaplan-Meier survival analysis for the occurrence of dementia in patients with PD are shown in Fig. 1. The dementia-free survival time from the onset of PD to the development of dementia in the PD population was 19.0 (14.0–29.0) years [median (interquartile range)]. Patients who were older at onset (≥62 years old) showed a shorter duration from the onset of PD to the development of dementia (log-rank test: p<0.001) (Fig. 2).
In the univariate analyses, age at onset, education period, and MMSE scores were significantly correlated with the development of dementia. However, there were no differences in dementia-free survival time between patients who had and did not have chronic illness. The sex of PD patients and the HY stage also did not affect dementia-free survival. In the multivariate analysis, age at onset and education period were found to be significant predictors of dementia in PD (Table 2).
There are several reasons why few studies have explored the frequency of dementia in PD using survival analyses from disease onset: 1) it is difficult to follow up a patient from disease onset to the late stages of PD, 2) dementia usually develops in the later stages of the disease and is very difficult to follow up—this typically leads to inaccurate information on the cumulative proportion of dementia because of a high dropout rate, and 3) dementia develops rather insidiously in PD, which makes it difficult to determine the time of dementia onset. These factors explain why serial assessment based on close observation is crucial for accurately determining event timing.
The median dementia-free survival time in our cohort was 19.0 years. While it is likely that this duration is longer than those found in previous studies, methodological differences make it difficult to directly compare the median dementia-free survival in our study with previous reports. In studies conducted in Sydney, 48% of patients who survived 15 years15 and 83% who survived 20 years6 were determined to have developed dementia. However, those studies did not provide information on the mean duration from the onset of PD to the development of dementia. One study that performed a survival analysis including the period from the onset of disease found that the estimated median duration from the onset of PD to the diagnosis of dementia was 12.1 years.7 However, those authors did not specify how they determined the time when dementia developed in the patients who had already developed dementia before the study. The longer median dementia-free survival time in the present study compared to the previous one could be partly attributed to the patients in our study being younger at onset (60.1 years vs. 65.4 years), especially given that we found the age at onset to be the most important risk factor for dementia. Another possible explanation is the exclusion of PD patients who might have developed dementia early, because only patients with a disease duration of more than 3 years were recruited in order to increase the diagnostic accuracy. It is likely that the application of strict criteria for the diagnosis of dementia in PD in our study also help to improve the reliability of our results.
Among the 119 patients who subsequently developed dementia, the mean duration from the onset of PD to the development of dementia was 10.6 years. This result is consistent with those of previous studies.616 We found wide variations, with some patients developing dementia approximately 10 years after onset of PD and half of our patients remaining free from dementia for 19 years, which raises the possibility that the risk factors for dementia vary between individual PD patients. We study found that the age at disease onset and the education period played important roles in the development of dementia in PD. Previous studies found that the importance of age as a risk factor for incident dementia was much more complex.1718 Most studies have found both age and the age at onset to be associated with a higher risk of dementia. This is plausible given that age is the most important risk factor for dementia in the general population. However, it can be difficult to disentangle their relative importance; that is, whether it is age or age at the onset of PD that is driving the age—associated risk of dementia. Aarsland et al.7 suggested that age-but not age at onset-is the key risk factor for dementia in PD. We unfortunately did not include a control group in our study, and so explored the independent effects of age and age at onset on the time to the development of dementia in PD.
Our study had several strengths. First, it had a longitudinal design and included a cohort with a large number of patients with PD. All patients were serially assessed at intervals of 3–6 months to determine the exact time when dementia developed. Second, the period from the onset of the illness was included to provide information on the duration from the onset of PD to the development of dementia, which has not been reported before. Third, the diagnostic accuracy of PD was increased because only patients with a disease duration of more than 3 years were included. However, this study also had several limitations. It is unlikely that our study cohort represented the whole PD population because it was a hospital-based study and most of the patients were referred to us from primary care units. Patients who might have developed dementia within 3 years after the onset of PD and who had dementia when visiting our clinic were excluded because the exact time when they developed dementia was unknown. Patients who had a disease duration of less than 3 years were also not included. These exclusions could reduce the generalizability of the study findings. The absence of an age-, sex-, and education-matched control group and the high dropout rate were other limitations of our study.
In conclusion, this hospital-based longitudinal study included a large number of patients with PD and has provided information on the duration from PD onset to the development of dementia in the PD population through serial assessment using survival analysis. Future studies without high dropout rates will be needed to obtain more reliable information on the median dementia-free survival time in the PD population.
Figures and Tables
Fig. 1
Kaplan-Meier curve for dementia-free survival in the whole cohort: estimated median dementia-free survival time from the PD onset to dementia was 19.0 years.

Fig. 2
Relationship between duration of PD and dementia diagnosis in patients with PD according to age at the onset of PD: older age of onset group (262 years old) had a higher proportion of dementia throughout the disease duration.

Table 1
Characteristics of the study subjects

Data are mean±standard deviation or n (%) values.
*Sum score of items 20 (tremor at rest), 22 (rigidity), 23 (finger taps), and 26 (leg agility) of Part 3 of the UPDRS, †Dose at the end of study, ‡Obtained using the Geriatric Depression Scale.
DM: diabetes mellitus, HY: Hoehn and Yahr, MMSE: Mini Mental State Examination, UPDRS: Unified Parkinson's Disease Rating Scale.
Table 2
Results from the Cox proportional-hazards regression models
References
2. Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord. 2005; 20:1255–1263.

3. de Lau LM, Schipper CM, Hofman A, Koudstaal PJ, Breteler MM. Prognosis of Parkinson disease: risk of dementia and mortality: the Rotterdam Study. Arch Neurol. 2005; 62:1265–1269.
4. Hobson P, Meara J. The detection of dementia and cognitive impairment in a community population of elderly people with Parkinson's disease by use of the CAMCOG neuropsychological test. Age Ageing. 1999; 28:39–43.

5. Levy G, Tang MX, Louis ED, CÔté LJ, Alfaro B, Mejia H, et al. The association of incident dementia with mortality in PD. Neurology. 2002; 59:1708–1713.

6. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008; 23:837–844.

7. Aarsland D, Kvaløy JT, Andersen K, Larsen JP, Tang MX, Lolk A, et al. The effect of age of onset of PD on risk of dementia. J Neurol. 2007; 254:38–45.

8. Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993; 50:140–148.

9. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 1998; 50:318 and 16 pages following.
10. Fahn S, Elton R. Members of the UPDRS Development Committee. Unified Parkinson's disease rating scale. In : Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Vol 2. Florham Park, NJ: Macmillan Health Care Information;1987. p. 153–163. p. 293–304.
11. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12:189–198.
12. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983; 17:37–49.

13. Kang Y, Na D, Hahn S. Seoul Neuropsychological Screening Battery. Incheon: Human Brain Research & Consulting Co.;2003.
14. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993; 43:2412–2414.
15. Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson's disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005; 20:190–199.

16. Hughes TA, Ross HF, Musa S, Bhattacherjee S, Nathan RN, Mindham RH, et al. A 10-year study of the incidence of and factors predicting dementia in Parkinson's disease. Neurology. 2000; 54:1596–1602.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download