Dear Editor,
A reversible corpus callosum lesion is a unique phenomenon with an unclear pathophysiology. Various etiologies may underlie the lesion, including toxins, encephalitis, influenza A, metabolic disorders such as hypernatremia, hypoglycemia, and vitamin deficiency disorders.1 In rare cases the lesion is caused by immunomodulating therapies.2 We report a patient with a reversible corpus callosum lesion associated with adalimumab treatment for ulcerative colitis (UC).
A 43-year-old man who had been diagnosed with UC 15 years previously was admitted due to an acute inflammatory lesion in his left inguinal area accompanied by abrupt-onset dysarthria. He had been managing the UC with naturopathy and regular colonoscopy checkups, and had not experienced a serious flare-up of the disease since its diagnosis. About 8 weeks before admission he had taken adalimumab for the first time to treat UC. The drug was administered at 40 mg for 2 days and then repeated 2 weeks later. He refused further adalimumab treatment due to severe insomnia related to the drug.
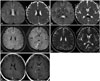
On admission, the patient was in a mildly confused state with definite dysarthria. His blood pressure was 148/89 mm Hg with a heart rate of 141 bpm and body temperature of 40.3℃. Laboratory tests revealed leukocytosis with elevated procalcitonin (PCT) (4.21 ng/mL) and C-reactive protein (CRP) (246.35 mg/L). The findings of other laboratory examinations, including liver function and electrolyte levels, were normal. Diffusion-weighted imaging (DWI) performed at admission showed a focal, well-defined lesion with restricted diffusion in the corpus callosum body and splenium (Fig. 1). The cerebrospinal fluid was normal. The patient was treated with empirical antibiotics due to an impression of sepsis caused by infection in the inguinal area. All of his symptoms improved dramatically within 4 days after admission. Magnetic resonance imaging performed 8 days after the initial DWI showed a markedly reduced lesion size without any regional enhancement. Evaluations of the inguinal lesion including a blood culture study all produced normal findings, and the patient was discharged without any symptoms.
The patient had no causative factors associated with a reversible corpus callosum lesion other than adalimumab treatment. Adalimumab is a recombinant human IgG1k anti-tumor necrosis factor (TNF)-α monoclonal antibody that is frequently used in the treatment of autoimmune-mediated inflammatory disorders. For many years it has been considered to be an effective treatment option for UC with fewer adverse effects. However, there have been some reports of CNS complications related to TNF-α inhibitors.2 In vitro studies have suggested that TNF-α inhibitors themselves can increase the permeability of the blood-brain barrier.34 TNF-α inhibitors share a common mechanism of action, but certain differences between specific drugs such as in their molecular structures and various pharmacologic characteristics may cause adverse manifestations.5 One of the possible pathomechanisms is inflammation by TNF-inhibitor-induced disruption of the balance between effector and regulatory T cells.6
The patient's inguinal cellulitis was one indication that inflammation had caused the corpus callosum lesion. The presence of a high fever with elevated PCT and CRP suggested infection due to the decreased immunity associated with the use of adalimumab. However, his symptoms and signs were dramatically improved after receiving antibiotics for only a few days. Blood cultures did not reveal any causative pathogen. Although PCT can be useful in differentiating bacterial infections, it can also be elevated in autoimmune-related disorders. A direct inflammatory reaction induced by the increased disease activity of UC was also considered, because treatment was not continued after adalimumab was stopped. However, there were no symptoms and signs suggesting flare-up of UC.
The time interval of 6 weeks before developing symptoms after terminating adalimumab treatment may be related to its long half-life of 2–3 weeks, which is the longest half-life of the TNF-α inhibitors. Delayed hypersensitivity may occur as an adverse drug event up to 3 weeks after exposure, but a longer interval is possible since it takes longer than 2 months to eliminate more than 95% of the drug from the body. The rapid improvement of neurologic symptoms in the present patient might have been related to a low drug concentration resulting from its short-term use. There are also previous reports of a very good prognosis for neurologic complications induced by a TNF-α inhibitor.26
The present case indicates that TNF-α inhibitors including adalimumab may cause delayed adverse reactions such as a reversible corpus callosum lesion.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download