Dear Editor,
Acute motor axonal neuropathy (AMAN) is an axonal variant of Guillain-Barré syndrome (GBS) that is distinguished from acute inflammatory demyelinating polyradiculoneuropathy (AIDP) by both the electrophysiological pattern and clinical presentation. Because AMAN selectively involves motor nerves, sensory and autonomic disturbances are generally not observed. Here we report an atypical case of AMAN that presented with sudden-onset quadriparesis that was worse in the lower extremities, severe acroparesthesia, abrupt-onset bladder dysfunction, and cerebrospinal fluid (CSF) pleocytosis.
A 53-year-old woman experienced severe diarrhea for 10 days that was followed by muscle weakness (grade 3 or 4 in the upper extremities and grade 1 or 2 in the lower extremities according to Medical Research Council criteria) and pain and numbness in all limbs. Difficulty with urination appeared suddenly and almost simultaneously. Cranial nerve function was intact. Deep tendon reflexes were normal in the arm but reduced in the legs. Pathological reflexes and sensory level could not be clearly observed. Blood laboratory findings were within normal ranges. CSF analysis revealed a red blood cell count of 5/mm3, a white blood cell count of 101/mm3 (comprising 86% lymphocytes and 14% monocytes), 67.1 mg/dL total protein, and 51 mg/dL glucose. Brain and spine MRI findings were unremarkable (Supplementary Fig. 1 in the online-only Data Supplement). Somatosensory-evoked potentials at the median and tibial nerves were also normal.
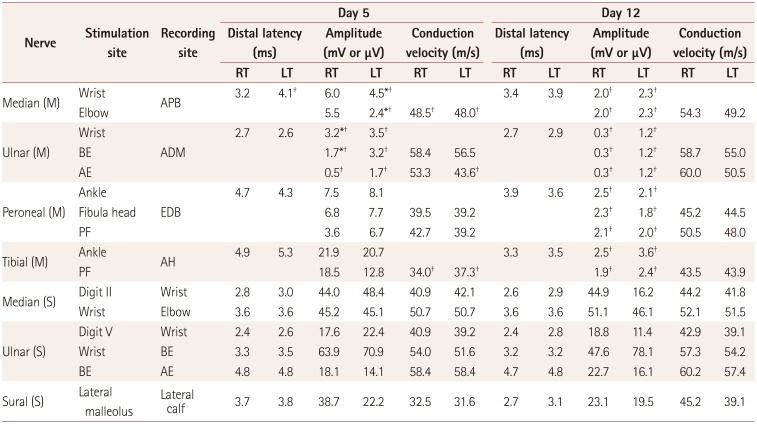
Nerve conduction studies performed on days 5 and 12 after the onset of weakness revealed reduced amplitudes in the distal nerves, with partial conduction block as well as mild slowing of the motor conduction velocity and prolonged distal latencies, which confirmed the diagnosis of AMAN (Table 1) (Supplementary Fig. 2 in the online-only Data Supplement). Furthermore, the finding of an enzyme-linked immunosorbent assay test for serum IgG autoantibodies to ganglioside GM1 was strongly positive (219.93%). The findings of extensive CSF and serological analyses for infectious diseases (syphilis, HIV, fungus, bacterium, and mycobacterium) and viral agents (herpes simplex, varicella zoster, cytomegalovirus, and Epstein-Barr virus) as well as CSF oligoclonal band and CSF malignant cell tests were all negative. The patient was treated with intravenous immunoglobulin, but her clinical improvement was slow.
AMAN is a motor-nerve-selective and axonal variant of GBS. The characteristic electrophysiological pattern is low-amplitude or absent compound muscle action potentials with normal sensory nerve action potentials and reversible conduction block. Unlike AIDP, AMAN rarely manifests with sensory loss, pain, paresthesia, or autonomic disturbance.1 The presence of certain symptoms such as bladder/bowel disturbance at symptom onset, the presence of a sensory level and CSF pleocytosis, and marked asymmetry of weakness cast doubt on a GBS diagnosis and may indicate a spinal cord lesion.2 This list of symptoms, which has been provided to clinicians to avoid misdiagnosis of GBS, should also be considered in the diagnosis of AMAN.
To the best of our knowledge, the present AMAN patient represents the first published case of AMAN mimicking a spinal cord lesion with pain, neurogenic bladder, and CSF pleocytosis. Only two cases of concomitant transverse myelitis and AMAN in one adult and one adolescent, respectively, have been reported previously.34 In these previous cases, sensory and bladder symptoms as well as CSF pleocytosis–which are atypical in AMAN–were due to concomitant myelopathy. In our case there was no evidence of spinal cord involvement in MRI, which suggests that the etiology of the atypical features was due to AMAN.
Our case implies that a patient with AMAN may exhibit several atypical manifestations that could lead to a misdiagnosis with spinal cord disease. Several studies therefore need to be applied to patients who present with the features outlined in this case in order to exclude diseases with similar presentations.
References
1. Kuwabara S, Yuki N. Axonal Guillain-Barré syndrome: concepts and controversies. Lancet Neurol. 2013; 12:1180–1188. PMID: 24229616.

2. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990; 27(Suppl):S21–S24. PMID: 2194422.

3. Saidha S, Renganathan R, Spillane J, McNamara B, Fanning N, Ryan AM. Simultaneous transverse myelitis and acute motor axonal neuropathy in an adult. J Neurol Neurosurg Psychiatry. 2008; 79:1302–1303. PMID: 18940993.

4. Howell KB, Wanigasinghe J, Leventer RJ, Ryan MM. Concomitant transverse myelitis and acute motor axonal neuropathy in an adolescent. Pediatr Neurol. 2007; 37:378–381. PMID: 17950429.

Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2017.13.2.205
Supplementary Fig. 1
MRI of the cervical spinal cord obtained with T2-weighted (A) and gadolinium enhancement (B). No abnormal signal intensity or enhanced lesion was observed.
Supplementary Fig. 2
Representative traces from nerve conduction studies performed on the right ulnar nerve at 5 days (A) and 12 days (B) after the onset of weakness.
Table 1
Results of nerve conduction studies (NCSs). The first NCS revealed (1) reduced CMAP amplitudes in the left median nerve and bilateral ulnar nerves with partial conduction block, (2) mild slowing of the motor conduction velocity in the bilateral median and tibial nerves, and (3) prolonged or absent F-wave and H-reflex latencies (data not shown). All of the sensory nerves were normal. Follow-up NCS showed remarkable CMAP amplitude reduction in all motor nerves (bilateral median, ulnar, peroneal, and tibial nerves) and still-preserved sensory nerve functions




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download