1. Yoo JH, Hunter J. Imaging spectrum of pediatric corpus callosal pathology: a pictorial review. J Neuroimaging. 2013; 23:281–295. PMID:
22273241.
2. Krupa K, Bekiesinska-Figatowska M. Congenital and acquired abnormalities of the corpus callosum: a pictorial essay. Biomed Res Int. 2013; 2013:265619. PMID:
24027754.

3. Bayram E, Topcu Y, Yis U, Cakmaci H, Kurul SH. Comparison of cranial magnetic resonance imaging findings and clinical features in patients with corpus callosum abnormalities. Neuropediatrics. 2014; 45:30–35. PMID:
23888465.

4. Lerman-Sagie T, Ben-Sira L, Achiron R, Schreiber L, Hermann G, Lev D, et al. Thick fetal corpus callosum: an ominous sign? Ultrasound Obstet Gynecol. 2009; 34:55–61. PMID:
19449354.

5. Koob M, Weingertner AS, Gasser B, Oubel E, Dietemann JL. Thick corpus callosum: a clue to the diagnosis of fetal septopreoptic holoprosencephaly? Pediatr Radiol. 2012; 42:886–890. PMID:
22006531.

6. Cavicchioni O, Gomes DM, Leroy B, Vialard F, Hillion Y, Selva J, et al. Prenatal diagnosis of de novo (7;19)(q11.2;q13.3) translocation associated with a thick corpus callosum and Wilms tumor of the kidneys. Prenat Diagn. 2005; 25:876–878. PMID:
16193462.
7. Rypens F, Sonigo P, Aubry MC, Delezoide AL, Cessot F, Brunelle F. Prenatal MR diagnosis of a thick corpus callosum. AJNR Am J Neuroradiol. 1996; 17:1918–1920. PMID:
8933878.
8. Pashaj S, Merz E, Wellek S. Biometry of the fetal corpus callosum by three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2013; 42:691–698. PMID:
23649512.

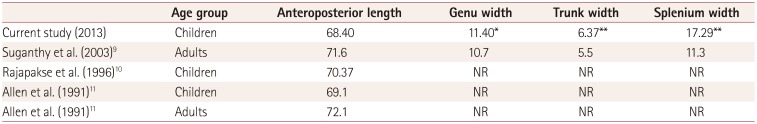
9. Suganthy J, Raghuram L, Antonisamy B, Vettivel S, Madhavi C, Koshi R. Gender- and age-related differences in the morphology of the corpus callosum. Clin Anat. 2003; 16:396–403. PMID:
12903061.

10. Rajapakse JC, Giedd JN, Rumsey JM, Vaituzis AC, Hamburger SD, Rapoport JL. Regional MRI measurements of the corpus callosum: a methodological and developmental study. Brain Dev. 1996; 18:379–388. PMID:
8891233.

11. Allen LS, Richey MF, Chai YM, Gorski RA. Sex differences in the corpus callosum of the living human being. J Neurosci. 1991; 11:933–942. PMID:
2010816.

12. Bermudez P, Zatorre RJ. Sexual dimorphism in the corpus callosum: methodological considerations in MRI morphometry. Neuroimage. 2001; 13(6 Pt 1):1121–1130. PMID:
11352617.

13. Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010; 30:10985–10990. PMID:
20720105.

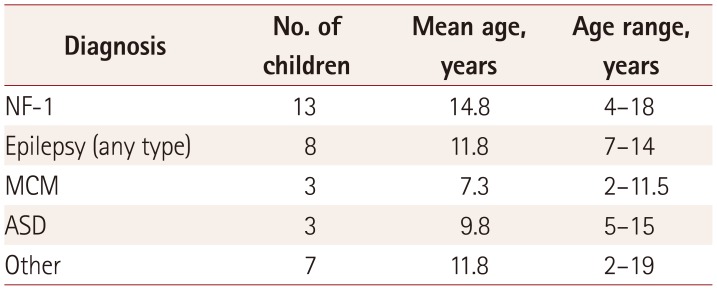
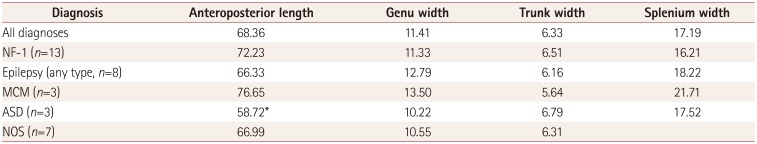
14. Mimouni-Bloch A, Kornreich L, Kaadan W, Steinberg T, Shuper A. Lesions of the corpus callosum in children with neurofibromatosis 1. Pediatr Neurol. 2008; 38:406–410. PMID:
18486822.

15. Margariti PN, Blekas K, Katzioti FG, Zikou AK, Tzoufi M, Argyropoulou MI. Magnetization transfer ratio and volumetric analysis of the brain in macrocephalic patients with neurofibromatosis type 1. Eur Radiol. 2007; 17:433–438. PMID:
16733674.

16. Conway RL, Pressman BD, Dobyns WB, Danielpour M, Lee J, Sanchez-Lara PA, et al. Neuroimaging findings in macrocephaly-capillary malformation: a longitudinal study of 17 patients. Am J Med Genet A. 2007; 143A:2981–3008. PMID:
18000912.

17. Boger-Megiddo I, Shaw DW, Friedman SD, Sparks BF, Artru AA, Giedd JN, et al. Corpus callosum morphometrics in young children with autism spectrum disorder. J Autism Dev Disord. 2006; 36:733–739. PMID:
16625438.

18. Booth R, Wallace GL, Happé F. Connectivity and the corpus callosum in autism spectrum conditions: insights from comparison of autism and callosal agenesis. Prog Brain Res. 2011; 189:303–317. PMID:
21489396.
19. Goldberg J, Szatmari P, Nahmias C. Imaging of autism: lessons from the past to guide studies in the future. Can J Psychiatry. 1999; 44:793–801. PMID:
10566110.

20. Göçmen R, Oğuz KK. Mega corpus callosum and caudate nuclei with bilateral hippocampal malformation. Diagn Interv Radiol. 2008; 14:69–71. PMID:
18553278.
21. Pöyhönen MH, Peippo MM, Valanne LK, Kuokkanen KE, Koskela SM, Bartsch O, et al. Hypertrichosis, hyperkeratosis, abnormal corpus callosum, mental retardation and dysmorphic features in three unrelated females. Clin Dysmorphol. 2004; 13:85–90. PMID:
15057123.
22. Hengst M, Tücke J, Zerres K, Blaum M, Häusler M. Megalencephaly, mega corpus callosum, and complete lack of motor development: delineation of a rare syndrome. Am J Med Genet A. 2010; 152A:2360–2364. PMID:
20803648.

23. Göhlich-Ratmann G, Baethmann M, Lorenz P, Gärtner J, Goebel HH, Engelbrecht V, et al. Megalencephaly, mega corpus callosum, and complete lack of motor development: a previously undescribed syndrome. Am J Med Genet. 1998; 79:161–167. PMID:
9788554.
24. Pride N, Payne JM, Webster R, Shores EA, Rae C, North KN. Corpus callosum morphology and its relationship to cognitive function in neurofibromatosis type 1. J Child Neurol. 2010; 25:834–841. PMID:
20142468.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download