1. Zaharchuk G, Olivot JM, Fischbein NJ, Bammer R, Straka M, Kleinman JT, et al. Arterial spin labeling imaging findings in transient ischemic attack patients: comparison with diffusion- and bolus perfusion-weighted imaging. Cerebrovasc Dis. 2012; 34:221–228. PMID:
23006669.

2. Chang JY, Kim DH, Chung JH, Yum KS, Hong JH, Han MK. Hospital-based prospective registration of acute transient ischemic attack and noncerebrovascular events in Korea. J Stroke Cerebrovasc Dis. 2015; 24:1803–1810. PMID:
26139456.

3. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000; 284:2901–2906. PMID:
11147987.

4. Purroy F, Begué R, Quílez A, Piñol-Ripoll G, Sanahuja J, Brieva L, et al. The California, ABCD, and unified ABCD2 risk scores and the presence of acute ischemic lesions on diffusion-weighted imaging in TIA patients. Stroke. 2009; 40:2229–2232. PMID:
19372450.

5. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995; 333:1581–1587. PMID:
7477192.
6. Kidwell CS, Alger JR, Di Salle F, Starkman S, Villablanca P, Bentson J, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke. 1999; 30:1174–1180. PMID:
10356095.

7. Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007; 370:1432–1442. PMID:
17928046.

8. Nah HW, Kwon SU, Kang DW, Lee DH, Kim JS. Diagnostic and prognostic value of multimodal MRI in transient ischemic attack. Int J Stroke. 2014; 9:895–901. PMID:
24256197.

9. Lamy C, Oppenheim C, Calvet D, Domigo V, Naggara O, Méder JL, et al. Diffusion-weighted MR imaging in transient ischaemic attacks. Eur Radiol. 2006; 16:1090–1095. PMID:
16395534.

10. Kim BJ, Kang HG, Kim HJ, Ahn SH, Kim NY, Warach S, et al. Magnetic resonance imaging in acute ischemic stroke treatment. J Stroke. 2014; 16:131–145. PMID:
25328872.

11. Qiao XJ, Salamon N, Wang DJ, He R, Linetsky M, Ellingson BM, et al. Perfusion deficits detected by arterial spin-labeling in patients with TIA with negative diffusion and vascular imaging. AJNR Am J Neuroradiol. 2013; 34:2125–2130. PMID:
23721895.

12. Shih LC, Saver JL, Alger JR, Starkman S, Leary MC, Vinuela F, et al. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke. 2003; 34:1425–1430. PMID:
12738899.

13. Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994; 36:557–565. PMID:
7944288.

14. Røhl L, Ostergaard L, Simonsen CZ, Vestergaard-Poulsen P, Andersen G, Sakoh M, et al. Viability thresholds of ischemic penumbra of hyperacute stroke defined by perfusion-weighted MRI and apparent diffusion coefficient. Stroke. 2001; 32:1140–1146. PMID:
11340223.

15. Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation. 2002; 105:656–662. PMID:
11827935.

16. Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994; 25:1847–1853. discussion 1853-1854. PMID:
8073468.

17. del Zoppo GJ, Schmid-Schönbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991; 22:1276–1283. PMID:
1926239.

18. del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003; 23:879–894. PMID:
12902832.

19. del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb Res. 2000; 98:73–81.

20. Liebeskind DS. Collateral circulation. Stroke. 2003; 34:2279–2284. PMID:
12881609.

21. Kang DW, Latour LL, Chalela JA, Dambrosia J, Warach S. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann Neurol. 2003; 54:66–74. PMID:
12838521.

22. Greif DM, Eichmann A. Vascular biology: brain vessels squeezed to death. Nature. 2014; 508:50–51. PMID:
24670635.
23. Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014; 508:55–60. PMID:
24670647.

24. Garcia JH, Kamijyo Y. Cerebral infarction. Evolution of histopathological changes after occlusion of a middle cerebral artery in primates. J Neuropathol Exp Neurol. 1974; 33:408–421. PMID:
4209682.
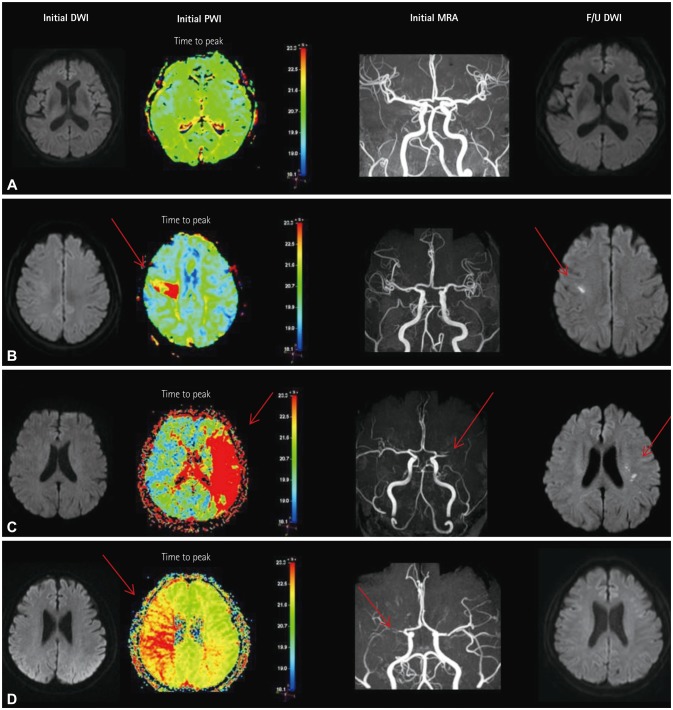

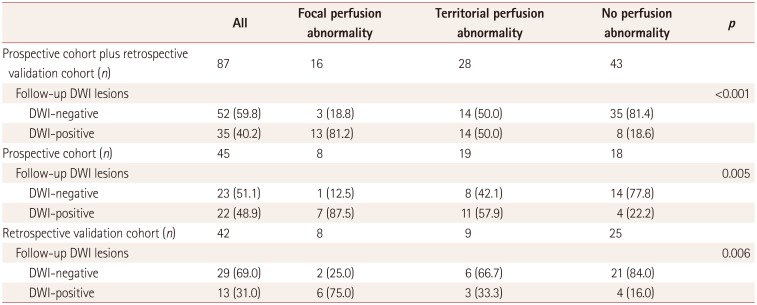
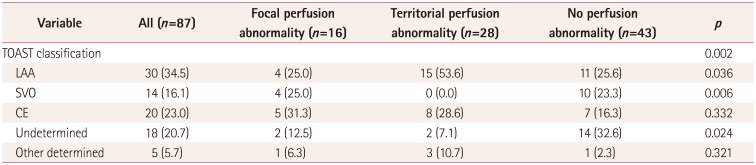
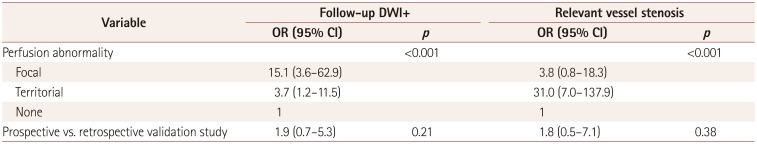





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download