Abstract
Background and Purpose
Measuring the extent of the collateral blood vessels using computed tomography (CT) angiography source images may promote tissue survival and functional gain in acute ischemic stroke patients who are candidates for endovascular recanalization treatment.
Methods
Of 5,558 acute stroke patients registered in a prospective clinical stroke registry, 104 met the selection criteria of endovascular recanalization treatment for internal cerebral artery or middle cerebral artery main-stem (M1) occlusions and presented for treatment ≤4 hours after the event. Using CT angiography source images, two independent and blinded reviewers measured the extent of collateral circulations at four regions, with good interrater reliability. The functional recovery at 3 months after stroke was used as an outcome variable.
Results
Cases with a sufficient collateral circulation at the Sylvian fissure showed significantly increased likelihood of having a modified Rankin Scale score of ≤2 at 3 months after stroke (adjusted odds ratio=3.03, 95% confidence interval=1.19–7.73, p=0.02), but the association became nonsignificant after adding the infarct volume to the model (p=0.65). The association between leptomeningeal convexity collaterals and functional recovery was no longer significant after adjusting for the infarct volume (p=0.28). The natural indirect effect of infarct volume on functional recovery was significant for both the Sylvian fissure (p=0.03) and leptomeningeal convexity (p=0.02) collaterals.
With a recent successful series of endovascular recanalization through stent-retrivers,12345 fast and unblemished recanalization of occluded cerebral arteries has become an achievable goal in the treatment of hyperacute ischemic stroke. Therefore, the latest clinical practice guideline recommends an endovascular recanalization strategy for an acute ischemic stroke of moderate severity if the intervention can be applied within 6 hours from last seen normal.6 However, stroke physicians worldwide are still waiting for a reasonable decision strategy to initiate this treatment in individual cases, since 29% to 59% of cases show profound functional dependence or mortality even after applying groundbreaking treatment. A strategy therefore needs to be developed for identifying the best candidates for endovascular recanalization treatment.
Currently, the suggested indications for endovascular recanalization include the baseline neurological severity, time delay from onset, and extent of irreversible infarction.6 However, these conventional indicators are representative characteristics only for those patients who show a favorable clinical response to treatment, and so they are of limited relevance to the condition of the ischemic brain in individual cases. Among the various advanced imaging techniques used to measure personalized ischemic/perfusion status, collateral imaging based on computed tomography (CT) angiography has been suggested as a good alternative due to its simplicity, speed, reliability, and practicality.7891011
The collateral circulation seems functionally inactive in the normal and stable brain, but it becomes therapeutically relevant in the treatment of acute ischemic stroke, since rescue cerebral blood flow may be perfused through alternative channels.1213 It is therefore reasonable to suppose that acute ischemic stroke patients with a sufficient collateral perfusion might have viable and reversible brain tissues-such patients may thus be good candidates for endovascular revascularization, which is inevitably delayed due to difficulties in logistics, the recruitment of intervention teams, and the accessibility of the occlusion site.14
In this context, the primary purpose of the present study was to determine the association between the baseline collateral status and stroke outcomes after endovascular recanalization, including functional recovery and the status of the radiological infarction. We also investigated the effects of the infarct volume on the initial collateral flow and the final functional status.
In total, 5,558 stroke patients were admitted to Seoul National University Bundang Hospital between July 2007 and February 2014. The authors identified analyzable cases from among them by applying the following inclusion criteria: 1) ischemic stroke with relevant infarction that was documented by relevant neuroimaging studies (n=4,045), 2) patient arriving within 4 hours since last seen normal (n=946), and 3) severe stenosis or occlusion in the internal carotid artery or proximal middle cerebral arteries including the main stem (M1) or secondary trunks (M2) (n=401). The following exclusion criteria were also applied: 1) missing modified Rankin Scale (mRS) score at 3 months after stroke (n=19), 2) no CT angiography evaluation or CT angiography being performed after the induction of intravenous thrombolysis (n=188), 3) hemodynamically irrelevant stenosis or a bilateral steno-occlusion that prevented measurement of the collateral status (n=46), or 4) no endovascular recanalization treatment (n=42). Application of the inclusion and exclusion criteria finally resulted in 104 cases being eligible for the current analysis. Acute stroke management (including hyperacute recanalization) was performed according to the current clinical practice guidelines for stroke care, institutional protocols, and at the discretion of individual physicians with direct liability.1516 This study was approved by the Institutional Review Board at Seoul National University Bundang Hospital (IRB approval no. B-1408-264-108).
We collected baseline demographic and clinical information for all study participants, including age, sex, body mass index, initial systolic and diastolic blood pressures, history of previous stroke, functional status before stroke, and cardiovascular risk factors such as hypertension (defined as previous use of antihypertensive medication, systolic blood pressure >140 mm Hg, or diastolic blood pressure >90 mm Hg at discharge), diabetes mellitus (defined as previous use of glucose-lowering medication or hemoglobin A1c ≥6.5%), hyperlipidemia (defined as previous use of lipid-lowering medication, fasting low-density lipoprotein cholesterol >160 mg/dL, or fasting total cholesterol >240 mg/dL), and habitual smoking (including current or past regular smoking).171819 We obtained laboratory data of the patients, including the initial levels of glucose, hemoglobin A1c, hemoglobin, total cholesterol, high-density lipoprotein cholesterol, triglycerides, and low-density lipoprotein cholesterol, and the leukocyte count and prothrombin time. The stroke characteristics included the time interval between the onset of symptoms and time of arrival, the National Institutes of Health Stroke Scale (NIHSS) score at admission, and treatment information. The functional status at 3 months after stroke was measured using the mRS score, which was determined through a structured telephone interview by an experienced physician assistant (M.-H. Yang).
Nonenhanced CT and CT angiography images were acquired according to standard departmental protocols with a 64- or 256-channel multidetector CT scanner (Brilliance 64 or Brilliance iCT, Philips Medical Systems, Eindhoven, the Netherlands). Nonenhanced CT was performed with the patient in a head holder in the transverse plane with minimal variations between scanners, and with scanning parameters of 120 kVp, 250 mAs, and 5-mm section thickness. CT angiography was performed with the administration of 80 mL of a nonionic contrast agent (Iomeron 350, Bracco Imaging Korea, Seoul, Korea) via an 18- or 21-gauge intravenous catheter at an injection rate of 3–5 mL/second using a power injector (Medrad Power Injector, Medrad, Indianola, PA, USA). The scanning parameters for 64-channel CT were 120 kVp, 250 mAs, 64-mm beam collimation, 0.5-second rotation time, 1-mm section thickness, and 0.985:1 pitch, while those for 256-channel CT were 80 kVp, 175 mAs, 128-mm beam collimation, 0.4-second rotation time, 1-mm section thickness, and 0.601:1 pitch. Images were obtained from the aortic arch through the skull vertex. The particular CT scanner used was randomly selected based on immediate availability.
Collateral status was measured primarily using a method proposed by Mass et al.8 Briefly, CT angiography source images of a 2-mm section were used to assess the presence of collateral vessels in the Sylvian fissure and leptomeningeal convexity separately. The collateral vessels in these two structures were graded in a comparison manner for the occluded hemisphere against the patent hemisphere as follows: 1, absent; 2, less than the patent contralateral side; 3, equal to the patent contralateral side; 4, greater than the patent contralateral side; and 5, exuberant. The anterior communicating arteries (A-comm) and posterior communicating arteries (P-comm) were also graded, as follows: 1, absent; 2, borderline present; 3, probably present; 4, definitely present; and 5, robust. The collateral status was separately categorized into insufficient (grade ≤2) and sufficient (grade ≥3). Two stroke neurologists performed the evaluation of the collateral status (J.-W. Chung and J.Y. Kim), and any disagreement was settled by discussion with a third reviewer (B.J. Kim). The reviewers were blinded to the clinical profiles, functional recovery, and infarction volume as measured using magnetic resonance imaging (MRI), but they were inevitably aware of the involved hemisphere and early ischemic changes detectable on the CT scan. The intraclass coefficient between the initial reviewers was 0.941 for Sylvian fissure collaterals, 0.971 for leptomeningeal convexity collaterals, 0.981 for A-comm, and 0.990 for P-comm.
The infarction volume was measured semiautomatically on the follow-up fluid-attenuated inversion recovery images of 84 available cases obtained 5–7 days after stroke onset (by J.-W. Chung) using MIPAV software (version 7.0.1, National Institutes of Health, Bethesda, MD, USA). The reperfusion status was assessed in postrecanalization conventional angiography images using the modified Thrombolysis in Cerebral Infarction (TICI) score20 by two reviewers (J.-W. Chung and H.-K. Park) who were blinded to the study purpose and clinical characteristics (kappa value=0.919 for a modified TICI score of ≥2b).
We analyzed differences between the groups using chi-squared tests for categorical variables and the independent-samples t-test or Fisher's exact test for continuous variables, as appropriate. Binary and ordinal logistic regression models were used to evaluate the association between the initial collateral status and the clinical outcomes at 3 months after stroke based on the mRS score and mortality rate. Due to the smallness of the sample, multivariable models were constructed in a stepwise manner by adding prespecified variables, and the number of covariates was limited to less than one-tenth of the number of outcome occurrences. The possible mediating factors were analyzed using a previously reported macro.2122 The cutoff for significance was set at p<0.05 in two-tailed tests. Statistical analyses were performed using Stata (version 14, StataCorp LP, College Station, TX, USA).
In total, 104 patients who arrived within 4 hours after last seen normal and who had received endovascular recanalization treatment were included in the analysis. Men comprised 53% of the sample, and the subjects were aged 68±13 years (mean±SD). The patients arrived at the hospital at a median of 57 minutes (interquartile range=38–89 minutes) after last seen normal, with a median NIHSS score of 16 points (interquartile range=12–20 points). On the initial CT angiography source images, the collateral circulation was sufficient in 47% and 49% of cases involving the Sylvian fissure and leptomeningeal convexity collateral vessels, respectively (Supplementary Table 1 in the online-only Data Supplement). After hyperacute treatment, successful recanalization to a modified TICI score of ≥2b was achieved in 89% of the cases; the mRS score was 0–2 at 3 months after stroke in 44% of the cases, but 15% of the patients had died at 3 months (Table 1). Among the indices for collateral circulation, A-comm and P-comm grades were irrelevant to collateral vessels at the Sylvian fissure and leptomeningeal convexity (Supplementary Table 2 in the online-only Data Supplement), and so these gradings were excluded from the analysis.
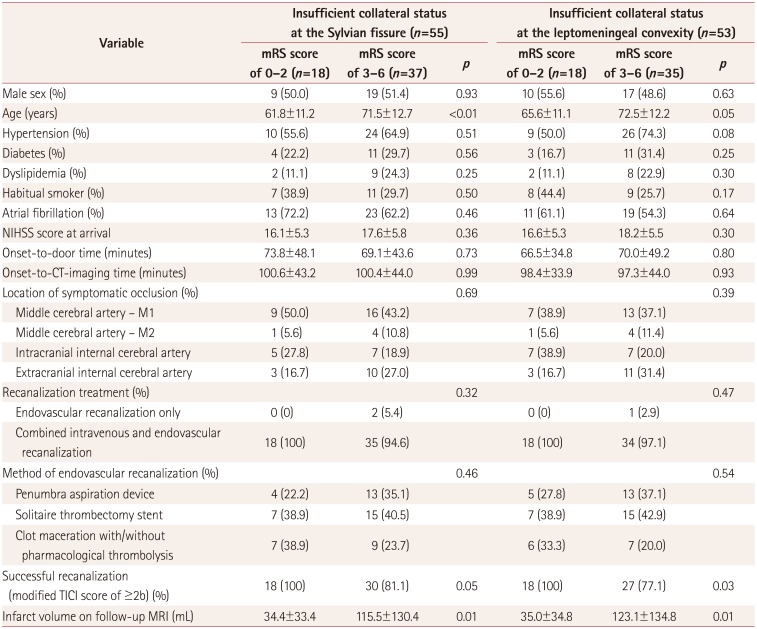
Regardless of the measurement location, the collateral circulations at the Sylvian fissure and leptomeningeal convexity showed comparable distributions of baseline clinical characteristics and outcome profiles (Table 2). Cases with a sufficient collateral circulation had a significantly lower NIHSS score at arrival and a lower volume of infarct tissue on follow-up MRI scans (which were available in only 84 cases). This resulted in the groups with a sufficient collateral circulation showing improved outcome indices at 3 months after stroke. Multivariate logistic models showed that a sufficient collateral circulation of the Sylvian fissure was favorably associated with a functional recovery and decreased mortality rate at 3 months after stroke after adjusting for the NIHSS score at arrival. However, this association consistently became nonsignificant when the infarct volume was added to the model (Table 3, Fig. 1). Additional bivariate analyses of subjects with an insufficient collateral circulation showed age and successful recanalization were two baseline characteristics that independently affected the functional recovery at 3 months after stroke (Table 4).
Due to the small number of available samples, the mediating effect of the infarct volume between the collateral status and clinical outcome was evaluated only for mRS scores of 0–2 at 3 months. When adjusted for age and male sex, the estimates (with 95% confidence intervals) of a controlled direct effect, a natural direct effect, a natural indirect effect, and a marginal total effect were 2.43 (0.57–10.36), 1.87 (0.62–5.61), 1.59 (1.05–2.40), and 2.97 (0.90–9.83), respectively, for the Sylvian fissure collaterals, and 2.56 (0.60–10.91), 1.65 (0.55–4.98), 1.63 (1.07–2.50), and 2.69 (0.83–8.76) for the leptomeningeal convexity collaterals.
From our analysis of 104 hyperacute ischemic stroke patients who were treated with an endovascular recanalization procedure, we found that a sufficient baseline collateral status measured at both the Sylvian fissure and leptomeningeal convexity was associated with a favorable clinical outcome after stroke. This association was partially mediated by the extent of the infarcted tissues measured at follow-up in MRI scans.
The latest clinical guideline for endovascular recanalization treatment of hyperacute ischemic stroke patients suggests that various imaging parameters (including the collateral status) require additional diagnostic information in order to be useful in clinical practice.6 However, it is reasonable to suggest that some endovascular cases can fall outside the scope of the guideline's recommendation, such as those with a milder neurological severity but a larger perfusion defect or a delayed arrival and a greater extent of viable tissues.23 Among the advanced imaging parameters, evaluating the collateral vessels on CT angiography source images has the following advantages: 1) it is a more widely available technique than MRI, 2) it does not require a time-consuming image reconstruction process, 3) it is noninvasive compared to angiography, 4) it shows higher interrater agreement, and 5) it offers a reliable measurement of the extent of collateral vessels and the prediction of clinical outcomes after stroke.1013242526 A recent clinical trial that incorporated a dynamic evaluation of CT angiography collaterals in the case selection procedure successfully demonstrated the efficacy of an endovascular intervention.3 The results of our study were consistent in showing that a sufficient baseline collateral perfusion may delay the evolution of an irreversible infarction over a median of 4.5 hours and thereby improve the clinical outcome of an endovascular-treated stroke.
The normal cerebral blood flow is more than 50 mL/100 g brain tissue/minute, and a reduced blood flow may lead to clinical signs of ischemia and irreversible death within minutes.272829 Additionally, in spite of a timely intervention, the recanalization can be futile in a substantial proportion of treated cases. Therefore, beyond the conventional indicators such as the time window and neurological severity, the stroke physician needs to know the vital status of ischemic tissues in the individual's brain. Most (89%) of our patients achieved an acceptable level of recanalization, and the intervention began at a median of 272 minutes after stroke onset. Because the baseline treatment profile was comparable between the groups with sufficient and insufficient collateral circulations, the disparate clinical and radiological outcomes could reasonably be attributed to the baseline collateral perfusion. Our results are consistent with a recent suggestion that reperfusion is superior to recanalization in determining the fate of ischemic tissues.30
A few limitation of our study should be considered. First, only a small number of patients were included and the analysis had a retrospective design. Second, for multiple clinical reasons, the final infarct volume was not measured in an MRI scan in every subject. Third, we were not able to determine whether a sufficient collateral circulation can sustain the ischemic brain or merely represents a restricted magnitude of the ischemic insult at the time of imaging. Fourth, although the site of occlusion may be an important determining factor of the collateral status, we did not analyze this further due to small number of subjects. Finally, we were not able to clarify all the possible mediating factors, such as the change in the infarct volume between before and after recanalization treatment.
This study found that a sufficient baseline collateral status in acute ischemic stroke patients was associated with improved functional recovery and decreased mortality rate at 3 months after stroke, and that this was partially mediated by a reduced volume of the final infarct tissue. It may be inferred from our results that advanced neuroimaging techniques and assessing the status of the collaterals at the Sylvian fissure and leptomeningeal convexity may be useful when deciding how to treat hyperacute ischemic stroke in individual patients. Since our study involved only a small number of cases, further studies with prospective designs and larger numbers of patients are warranted.
References
1. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015; 372:2296–2306. PMID: 25882510.

2. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015; 372:2285–2295. PMID: 25882376.

3. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030. PMID: 25671798.
4. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusionimaging selection. N Engl J Med. 2015; 372:1009–1018. PMID: 25671797.

5. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20. PMID: 25517348.
6. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015; 46:3020–3035. PMID: 26123479.

7. Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009; 132(Pt 8):2231–2238. PMID: 19509116.

8. Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009; 40:3001–3005. PMID: 19590055.

9. Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010; 41:2316–2322. PMID: 20829514.

10. Bhatia R, Bal SS, Shobha N, Menon BK, Tymchuk S, Puetz V, et al. CT angiographic source images predict outcome and final infarct volume better than noncontrast CT in proximal vascular occlusions. Stroke. 2011; 42:1575–1580. PMID: 21566239.

11. Menon BK, Smith EE, Modi J, Patel SK, Bhatia R, Watson TW, et al. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR Am J Neuroradiol. 2011; 32:1640–1645. PMID: 21799045.

12. Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke. 2003; 34:2750–2762. PMID: 14576375.
13. Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011; 10:909–921. PMID: 21939900.

14. Liebeskind DS, Tomsick TA, Foster LD, Yeatts SD, Carrozzella J, Demchuk AM, et al. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke. 2014; 45:759–764. PMID: 24473178.

15. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013; 44:870–947. PMID: 23370205.
16. Hong KS, Ko SB, Yu KH, Jung C, Park SQ, Kim BM, et al. Update of the Korean clinical practice guidelines for endovascular recanalization therapy in patients with acute ischemic stroke. J Stroke. 2016; 18:102–113. PMID: 26846761.

17. Whitworth JA. World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003; 21:1983–1992. PMID: 14597836.
18. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012; 35(Suppl 1):S64–S71. PMID: 22187472.
19. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002; 106:3143–3421. PMID: 12485966.
20. Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, et al. Revascularization results in the interventional management of stroke II trial. AJNR Am J Neuroradiol. 2008; 29:582–587. PMID: 18337393.

21. Valeri L, VanderWeele TJ. Mediation analysis allowing for exposuremediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013; 18:137–150. PMID: 23379553.

22. VanderWeele TJ. Explanation in Causal Inference : Methods for Mediation and Interaction. New York: Oxford University Press;2015.
23. Kim BJ, Han MK, Park TH, Park SS, Lee KB, Lee BC, et al. Trends in the effectiveness of endovascular recanalization for acute stroke: is a change taking place. J Stroke Cerebrovasc Dis. 2015; 24:866–873. PMID: 25726023.

24. McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol. 2012; 33:576–582. PMID: 22135128.

25. Calleja AI, Cortijo E, García-Bermejo P, Gómez RD, Pérez-Fernández S, Del Monte JM, et al. Collateral circulation on perfusion-computed tomography-source images predicts the response to stroke intravenous thrombolysis. Eur J Neurol. 2013; 20:795–802. PMID: 23278976.

26. Lum C, Ahmed ME, Patro S, Thornhill R, Hogan M, Iancu D, et al. Computed tomographic angiography and cerebral blood volume can predict final infarct volume and outcome after recanalization. Stroke. 2014; 45:2683–2688. PMID: 25104844.

27. Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest. 1948; 27:476–483. PMID: 16695568.
28. Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia-the ischemic penumbra. Stroke. 1981; 12:723–725. PMID: 6272455.
29. Jones TH, Morawetz RB, Crowell RM, Marcoux FW, FitzGibbon SJ, DeGirolami U, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981; 54:773–782. PMID: 7241187.

30. Cho TH, Nighoghossian N, Mikkelsen IK, Derex L, Hermier M, Pedraza S, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke. 2015; 46:1582–1589. PMID: 25908463.

Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2017.13.2.121.
Fig. 1
Illustrative cases depicting the collateral status. CBF: cerebral blood flow, MTT: mean transit time.
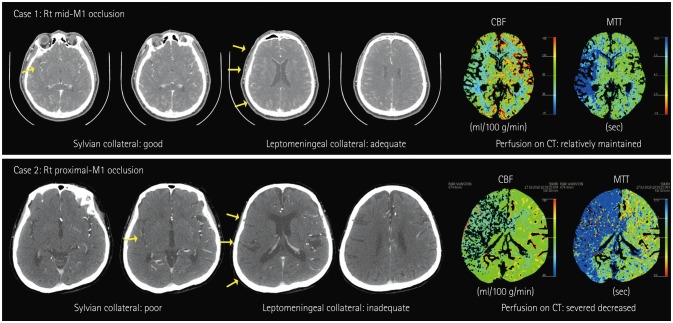
Table 1
Baseline characteristics (n=104)
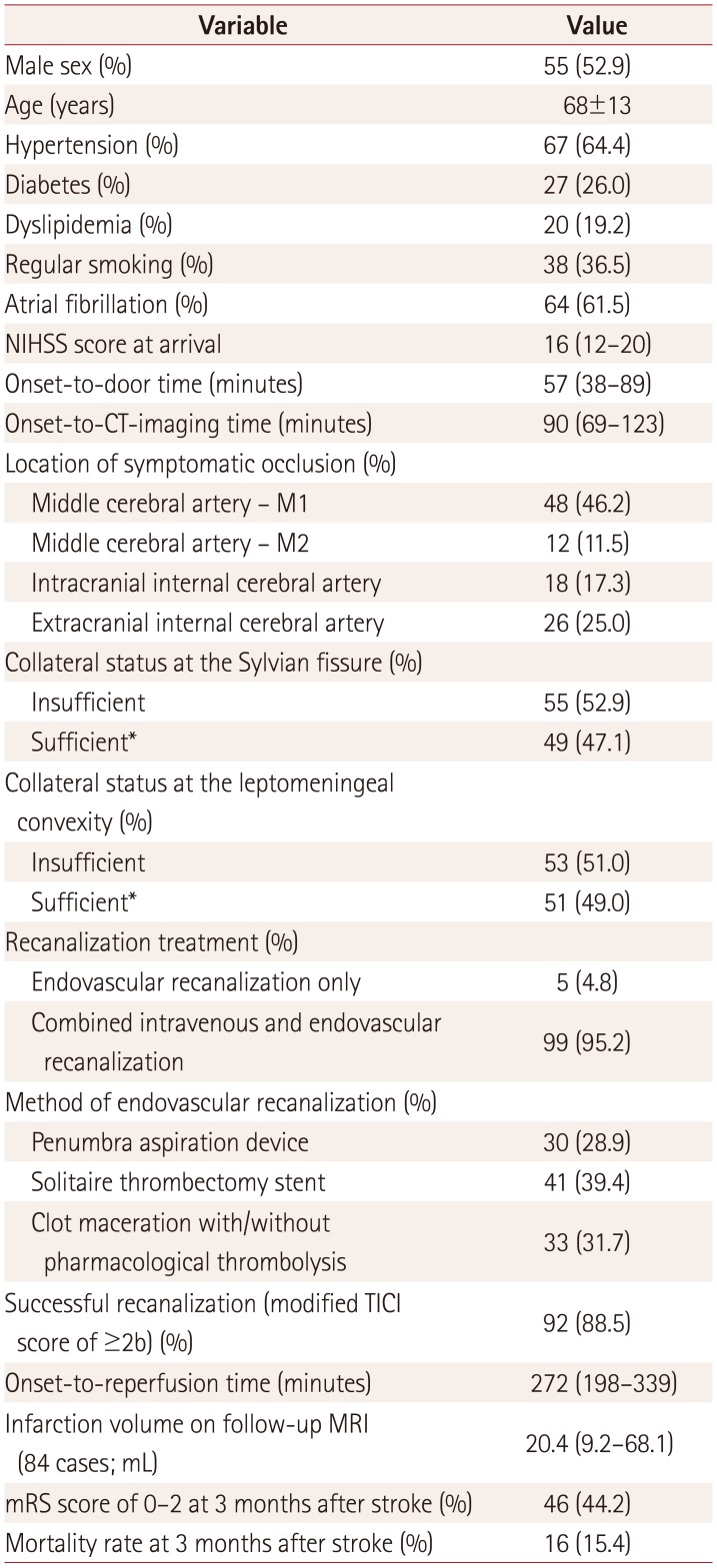
Table 2
Results of bivariate analyses according to the pretreatment collateral status in CT angiography
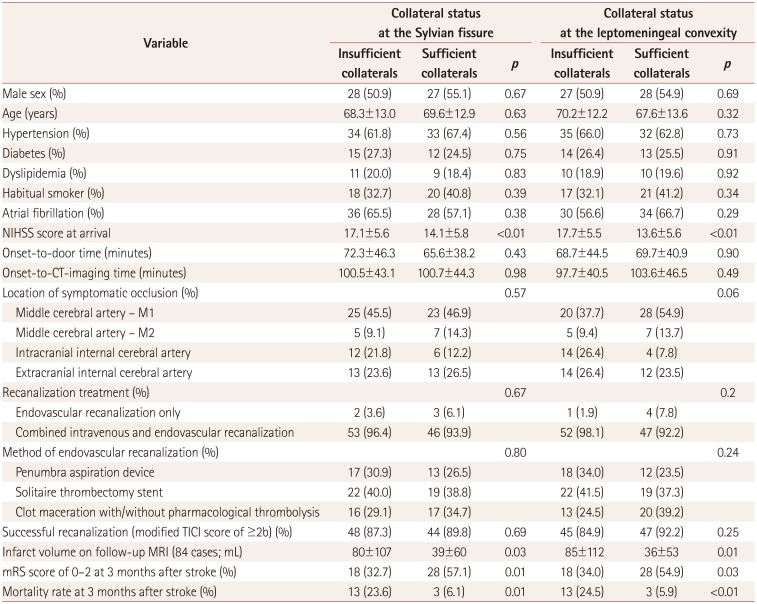
Table 3
Results of multivariable analyses
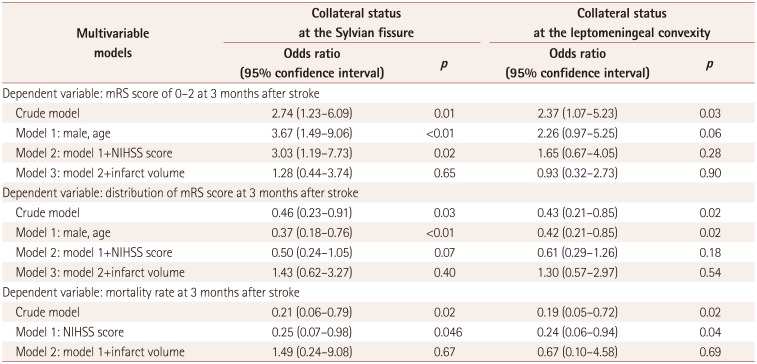
Table 4
Results of bivariate analyses according to favorable functional recovery at 3 months after stroke among subjects with an insufficient collateral circulation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download