Dear Editor,
The role of immune dysregulation in neurological disorders has been well recognized and is widely accepted. Limbic encephalitis (LE) has been associated with several antibodies (Abs) covering a wide clinical spectrum, with new entities continually being recognized. The patients are usually young and in the most productive years of their lives, which emphasizes the need for early diagnosis and prompt treatment.
We present a case of a 34-year-old male presenting with rapidly progressive memory loss and behavioral disturbances that had first appeared 4 months previously. This was accompanied with frequent, involuntary, and dystonic movements of the face and either upper limb for 2 months. The results of a general physical examination and systemic examination were normal. A neurological examination revealed significantly impaired frontal lobar functions and impaired working, recent, and episodic memory functions, with a Mini Mental State Examination (MMSE) score of 16 (out of 30) at the time of admission. The movements were stereotypical, with rapidly alternating dystonic movements of either limbs and face, consistent with faciobrachial dystonic seizures (FBDS). The other findings of the neurological and systemic examinations were unremarkable.
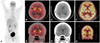
The results of blood investigations including hemogram, serum sodium and other electrolytes, routine biochemistry, viral markers (including retroviral serology), serum venereal disease research laboratory test, vasculitic markers, and thyroid profile, and a complete cerebrospinal fluid (CSF) analysis were normal. Due to a strong clinical suspicion of autoimmune encephalitis, magnetic resonance imaging (MRI) of the brain was performed, for which the findings were unremarkable (Fig. 1C and E). No abnormality was detected in contrast-enhanced computed tomography of the chest and abdomen. Whole-body 18F fluoro-2-deoxy-D-glucose (F-18 FDG) positron-emission tomography (PET) demonstrated bilateral striatal hypermetabolism (Fig. 1A, B, and D). The serum of the patient tested positive for leucine-rich glioma inactivated-1 (LGI-1) Ab, but CSF was not tested for this Ab. Although the patient was refractory to antiepileptic drug therapy, he responded remarkably to methylprednisolone pulse therapy. At a 6-month follow-up the patient was seizure-free, had an MMSE score of 30, and had returned to performing his routine work. Repeat F-18 FDG-PET showed complete reversal of the previously seen striatal hypermetabolism (Fig. 1F and G).
LGI-1-Ab-mediated LE has a specific seizure semiology of FBDS, which are reportedly highly refractory to antiepileptic drugs.1 Neuroimaging in patients with paraneoplastic LE may reveal uni- or bilateral T2-weighted hyperintense lesions in the medial temporal lobes.2 The findings of brain MRI in LGI-1-associated LE, at the stage of FBDS, are typically unremarkable, though there have been isolated cases of T2-weighted hyperintense signal alterations, diffusion restriction, and contrast-enhanced lesions of the basal ganglia.3 F-18 FDG-PET imaging is highly sensitive for detecting abnormalities in LE.4 In patients with bilateral FBDS without cognitive deficits, bilateral striatal hypermetabolism has been observed in F-18 FDG-PET even in the absence of basal ganglia abnormalities in MRI. The presence of striatal hypermetabolism in F-18 FDG-PET images is strongly indicative of LE and can help in diagnosing this treatable condition.5
In the present case, a history of typical FBDS, strong clinical suspicion of LE, and typical changes on PET were instrumental in diagnosing a treatable condition. The patient exhibited a remarkable response to immunotherapy (methylprednisolone pulse) therapy. LE is a reversible cause of dementia and a significant social burden. The normalization of metabolism in the follow-up brain PET images shows that such images can be reliably used for monitoring patients with LGI-1 encephalitis.
Figures and Tables
Fig. 1
18F-FDG PET/CT whole body scan acquired after standard protocol, maximum intensity projection image (A) show abnormal increased FDG uptake in bilateral basal ganglia regions and no hyper metabolism elsewhere in the body. Axial (B and C) and coronal (D and E) sections with fused CT images show diffusely increased FDG uptake (arrow) in bilateral basal ganglia suggesting striatal hyper-metabolism on both sides. Axial (F) and coronal (G) sections with fused CT images show normal FDG uptake on 6 months follow up. 18F-FDG: 18F fluoro-2-deoxy-D-glucose.
References
1. Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, et al. Faciobrachial dystonic seizures precede LGI1 antibody limbic encephalitis. Ann Neurol. 2011; 69:892–900.

2. Urbach H, Soeder BM, Jeub M, Klockgether T, Meyer B, Bien CG. Serial MRI of limbic encephalitis. Neuroradiology. 2006; 48:380–386.

3. Irani SR, Stagg CJ, Schott JM, Rosenthal CR, Schneider SA, Pettingill P, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain. 2013; 136(Pt 10):3151–3162.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download