Dear editor,
Lesions involving the hypothalamus with various etiologies such as tumors, stroke, neurosarcoidosis, Wernicke's encephalopathy, encephalitis, traumatic brain injury, and hydrocephalus can disrupt normal thermoregulation. There have been rare reports of demyelination in the hypothalamus presenting with dysregulated thermoregulation. For instance, hypothermia has been reported in multiple sclerosis, with postmortem autopsy confirming the presence of hypothalamic demyelination.12 In this study we observed a rare case of aquaporin-4 (AQP4)-antibody-positive neuromyelitis optica spectrum disorder (NMOSD) presenting with prolonged hyperthermia.
A 26-year-old woman presented with persistent high fever and irregular menstrual cycle for 2 weeks followed by hypersomnolence (more than 10 hours of sleep per day) and memory impairment. She had a past history of encephalomyelitis 2 years previously that developed after an episode of gastrointestinal infection, with complete recovery (Supplementary Fig. 1 in the online-only Data Supplement). She developed increased appetite 3 days prior to the present admission. There was no neck stiffness, joint pain, skin rash, abdominal pain, or symptoms of respiratory or urinary tract infection. On admission, fever persisted despite the administration of acetaminophen (500 mg every 6 hours). The findings of a neurologic examination were unremarkable except for poor attention and impaired short-term memory. A full septic screen including white blood cell count, C-reactive protein, erythrocyte sedimentation rate, urinary analysis, chest X-ray, and cerebrospinal fluid study produced normal results. Other laboratory investigations demonstrated mild hypoosmolar (264 mOsm/L) hyponatremia (128 mmol/L). Endocrinologic assays showed hyperprolactinemia (30.350 ng/mL) as well as a decreased luteinizing hormone (LH) level (0.310 mIU/mL). Her autoimmune profile and tumor markers were unremarkable.
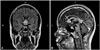
Electroencephalography performed on day 2 of admission revealed diffuse theta activities without epileptiform discharge. The results of a fundus examination and visual evoked potentials were normal. Magnetic resonance imaging (MRI) performed on day 3 demonstrated T2-weighted MRI hyperintense lesions with gadolinium-enhancement involving the anterior hypothalamus, optic chiasm, mammillary bodies, and septum pellucidum (Fig. 1).
The patient was treated with intravenous pulse methylprednisolone at 1 g daily for 5 days, followed by a short course of oral prednisolone (initially 60 mg daily). Her hypersomnolence and short-term memory impairment improved significantly after 2 days of pulse therapy with hyperthermia, and these conditions had resolved completely at the completion of treatment. She was discharged 1 week later without complications. One month later her serum AQP4 antibody level as detected using an enzyme-linked immunosorbent assay was 22.2 U/mL (<3.0 U/mL in the normal population). A diagnosis of NMOSD was thus made according to the core clinical and neuroimaging characteristics and her seropositivity for AQP4 antibody.3 Azathioprine (100 mg per day) was prescribed for long-term maintenance.
Antibodies to AQP4 constitute a sensitive and highly specific serum marker for the diagnosis of NMOSD. Despite the strong expression of AQP4 protein in the hypothalamus, NMOSD with hypothalamic involvement is uncommon (2.5–3% of NMOSD cases)45 but could be the initial presentation.
Various symptoms caused by NMOSD with hypothalamic involvement such as endocrinopathy, autonomic dysfunction, sleep-cycle disturbance, and dysregulated thermoregulation can be obscure and easily underrecognized. Indeed, the prolonged and unexplained hyperthermia of our patient during the initial 2 weeks as the only clue to hypothalamic involvement could easily have lead us to a misdiagnosis. The medial preoptic/anterior hypothalamus area (POA) plays a critical role in thermoregulation.6 The strongly enhanced T1-weighted MRI lesion in the POA of our patient indicates an active inflammatory process, which may result in an elevated set-point for core temperature.
While the underlying mechanism is poorly understood, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) in patients with NMOSD is not uncommon, especially in cases with hypothalamus involvement.7 The complete resolution of euvolemic hypoosmolar hyponatremia in our patient after pulse therapy suggests SIADH as the cause of hyponatremia. Hyperprolactinemia has been described in opticospinal multiple sclerosis, whereby the tuberoinfundibular dopaminergic neurons damaged by an inflammation reaction would lead to uninhibited prolactin production.8 The release of LH is regulated by neurons expressing the gonadotropin-releasing hormone that are located at the preoptic hypothalamus. A decrease in LH level may therefore reflect hypothalamic dysfunction.
In conclusion, we report a rare case of AQP4-antibody-positive NMOSD presenting with steroid-responsive hyperthermia. NMOSD can present as fever of unknown origin in young women with or without other hypothalamic symptoms, and hence prompt workup for NMOSD is needed to avoid treatment delay.
Figures and Tables
References
1. Edwards S, Lennox G, Robson K, Whiteley A. Hypothermia due to hypothalamic involvement in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1996; 61:419–420.

2. White KD, Scoones DJ, Newman PK. Hypothermia in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1996; 61:369–375.

3. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015; 85:177–189.

4. Chan KH, Tse CT, Chung CP, Lee RL, Kwan JS, Ho PW, et al. Brain involvement in neuromyelitis optica spectrum disorders. Arch Neurol. 2011; 68:1432–1439.

5. Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006; 63:964–968.

6. Benarroch EE. Thermoregulation: recent concepts and remaining questions. Neurology. 2007; 69:1293–1297.

Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.3988/jcn.2016.12.4.515.
Supplementary Fig. 1
A: Sagittal T2-weighted MRI illustrating hyperintensities in the medulla and upper cervical cord. B: Axial fluid-attenuated inversion-recovery sequence showing inflammation process involving the periependymal region of the brainstem (arrow), which was highly specific for neuromyelitis optica spectrum disorder. C: T1-weighted MRI following gadolinium administration illustrating no significant enhancement.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download