Dear Editor,
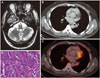
A 48-year-old male had been apparently healthy until 3 months previously when he developed difficulty in walking. This progressed rapidly, and 1 month later he additionally developed dysarthria and tremulousness of both upper limbs. At presentation he had a broad gait, impaired tandem walking, titubation, scanning speech, intention tremors, gaze-evoked nystagmus, hypometric saccades, broken pursuits and hypotonia in all limbs. There were no other focal neurological deficits. Detailed medical history-taking including drug and toxin exposure revealed no contributing factors. He was evaluated for rapidly progressive cerebellar ataxia, and the differential diagnosis was considered postinfectious demyelination, cerebellar space-occupying lesions, immune-mediated ataxia including gluten ataxia, ataxia associated with antibodies against glutamic acid decarboxylase (anti-GAD), steroid-responsive encephalopathy associated with autoimmune thyroiditis, and paraneoplastic cerebellar degeneration. Magnetic resonance imaging (MRI) of the brain showed mild prominence of bilateral cerebellar folia (Fig. 1A). The findings of investigations that included an antinuclear antibody panel, angiotensin-converting enzyme, thyroid function tests including a thyroid peroxidase antibody, serum venereal disease research laboratory test, vitamin B12, and IgA tissue transglutaminase were normal. The levels of anti-GAD antibody, vitamin B1, and vitamin E were not measured. His visual and auditory evoked potentials and the findings of nerve conduction studies were normal. The cerebrospinal fluid (CSF) protein concentration was 56 mg/dL (normal 14–45 mg/dL), and the CSF cell count, glucose levels, cytology for malignant cells, and cultures for infectious diseases did not reveal any contributing factors. A computed tomography (CT) scan of the chest and fluorodeoxyglucose positron-emission tomography showed an irregular heterogeneous soft-tissue mass lesion in the left lower lobe of the lung that was abutting the left upper bronchus lobe, the superior division of the left lower lobe of the bronchus, and major vessels (Fig. 1B and C). All of tests with a paraneoplastic panel of antibodies [anti-antineuronal nuclear antibody 1, 2, and 3; anti-Purkinje cell antibody 1 and 2; anti-Tr, antiglial nuclear antibody 1, antiamphyphsin, anti-collapsin response mediator protein 5, and anti-Ma/Ta] was negative. Histopathology of the mass revealed monomorphic tumor cells with a high nuclear-to-cytoplasm ratio arranged in nests along with a salt-and-pepper chromatin appearance without any necrosis, an appearance is suggestive of a carcinoid tumor (Fig. 1D). He underwent left pneumonectomy without no complications. Considering paraneoplastic cerebellar degeneration, he was treated with monthly intravenous methylprednisolone and intravenous immunoglobulin (IVIG) at 2 g/kg for 6 months. He improved symptomatically with reductions in ataxia and tremor, and became ambulant with support. This patient responded well to removal of the tumor and immunotherapy without no worsening at a 2-year follow-up.
This report represents a unique case of subacute paraneoplastic cerebellar degeneration associated with a bronchial carcinoid. Carcinoid tumors more commonly cause endocrine paraneoplastic syndromes such as Cushing syndrome, acromegaly, hypercalcemia, and hypoglycemia.1 Paraneoplastic neurological syndromes associated with carcinoid tumors are rare, which include Lambert-Eaton myasthenic syndrome, limbic encephalitis, and autonomic dysfunction.1 Balducci et al.2 described a case of paraneoplastic cerebellar degeneration associated with a malignant gastric carcinoid. Paraneoplastic cerebellar degeneration is commonly associated with small-cell lung carcinomas, breast and ovarian carcinomas, and lymphomas.3
Paraneoplastic syndrome is strongly suspected clinically when there is a subacute onset and progressive course of symptoms leading to severe disability. Nearly 50% of cases of subacute cerebellar degeneration have a paraneoplastic etiology.4 The underlying pathophysiology for cerebellar damage is mediated by the immune system, with several antibodies being implicated (anti-Yo, anti-Hu, anti-Tr, anti-Ri, and anti-mGluR1).56 Subacute cerebellar degeneration associated with lung cancer is commonly associated with anti-Hu, anti-Ri, anti-Tr, and P/Q-type anti-voltage-gated calcium-channel antibodies. Most of these patients are left with significant deficits due to cerebellar damage unless an early diagnosis is made and treatment started at an early stage. The treatment involves removal of the primary tumor and IVIG or plasmapheresis.3




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download