Abstract
Background and Purpose
This study evaluated the outcome following surgery for carotid artery stenosis in a single institution during a 10-year period and the relevance of aging to access to surgery.
Methods
Between January 2001 and December 2010, 649 carotid endarterectomies (CEAs) were performed in 596 patients for internal carotid artery occlusive disease at our institution; 596 patients received unilateral CEAs and 53 patients received bilateral CEAs. Data regarding patient characteristics, comorbidities, stroke, mortality, restenosis, and other surgical complications were obtained from a review of medical records. Since elderly and high-risk patients comprise a significant proportion of the patient group undergoing CEAs, differences in comorbidity and mortality were evaluated according to age when the patients were divided into three age groups: <70 years, 70-79 years, and ≥80 years.
Results
The mean age of the included patients was 67.5 years, and 88% were men. Symptomatic carotid stenosis was observed in 65.7% of patients. The rate of perioperative stroke and death (within 30 days of the procedure) was 1.84%. The overall mortality rate was higher among patients in the 70-79 years and >80 years age groups than among those in the <70 years age group, but there was no significant difference in stroke-related mortality among these three groups.
Stroke is the second leading cause of death after cancer in Korea. According to the 2005 Korean National Health and Nutrition Examination Survey, the age-standardized prevalence of stroke diagnosed by physicians was 15.9 per 1,000 (men, 16.44 per 1,000; women, 15.39 per 1,000). When stratified according to age, the stroke prevalence increases after the sixth decade of life: for every 1,000 people, it is 6.53, 24.26, 57.96, and 67.45 in the fifth, sixth, seventh, and eighth decades, respectively. The estimated stroke incidence was 216 per 100,000 person-years based on the 2004 insurance claim database of the Korean Health Insurance Review Agency and national death certificate data. According to an analysis of data from the insurance claim database of the Korean Health Insurance Review Agency, ischemic stroke accounted for 76.1% of cases and hemorrhagic stroke 23.9% in 2009.1 Stenosis of the carotid artery is a major cause of stroke and other adverse neurological events. The effectiveness of open carotid endarterectomy (CEA) in reducing the risk of stroke in selected patients with carotid artery stenosis has been established since the first successful procedure was carried out in 1954.23 In several large reviews, the combined risk of perioperative stroke or death in patients undergoing CEA was approximately 3% for asymptomatic and 5-6% for symptomatic stenosis.456 Carotid artery stenting (CAS) may be performed in high-risk patients with carotid stenosis, but CEA remains the standard of care, even in high-risk surgical patients.
Our experience and outcomes with CEA in carotid stenosis over a 10-year period are presented herein. The aim of this study was to establish whether these CEA outcomes concur with those presented in large international trials, and to determine whether CEA is a safe procedure in elderly and high-risk patients.
The medical records from a hospital database of 596 consecutive patients who underwent CEA over a 10-year period (January 2001 to December 2010) at our institute were reviewed retrospectively. Demographic details, comorbidities, stroke, mortality, restenosis, cause of death, and other surgical complications were analyzed for patients who underwent CEA during the study period. The operative criterion for eligibility for CEA was symptomatic >60% stenosis or asymptomatic >70% stenosis of the internal carotid artery (ICA) according to neurological symptomatology, where the degree of stenosis was determined on a carotid duplex scan. In symptomatic carotid stenosis, most patients have complained of at least two kinds or complex symptoms. If the patient complained of at least two kinds of symptoms, all symptoms were checked. Nonspecific symptoms (e.g., dizziness, headache, paresthesia, and tremor) were classified separately.
Bilateral carotid stenosis was managed with staged endarterectomy. In symptomatic patients, CEA was initially performed for the symptomatic side, and asymptomatic patients initially received CEA for the side with higher-grade carotid stenosis. At the onset of the study, patients received contralateral CEA a few weeks after the initial CEA. However, in the later period of the study, closely staged bilateral CEAs were performed with an intersurgical period of 7 days. Only the first CEA case was included when assessing the mortality rate for patients who underwent bilateral CEA. Differences in comorbidity and mortality were analyzed according to age after dividing the patients into three age groups: <70 years, 70-79 years, and ≥80 years. Patients were also divided into two groups according to the American Society of Anesthesiologists (ASA) physical status classification system to evaluate differences in mortality according to each patient's physical status. Patients with an ASA score of ≥3 were considered "high risk," while those with an ASA score of ≤2 were considered "low risk." This study was approved by the Institutional Review Board of our institution.
Diagnostic studies included preoperative carotid duplex ultrasound scanning and either magnetic resonance angiography (MRA) or conventional cerebral angiography. The preoperative diagnostic imaging studies performed varied over the 10-year study period, and conventional cerebral angiography was performed only in the late period of this study in cases of disagreement between two other diagnostic imaging studies. Antiplatelet medications were continued and not stopped during the perioperative period. The surgical procedures were preferably carried out under regional cervical block (superficial and deep), with general anesthesia with endotracheal intubation only used selectively in patients who were expected not to tolerate ICA clamping on preoperative MRA.7
Systemic heparinization was administered to all patients (80 IU/kg up to 5,000 IU, intravenously) before carotid clamping. In patients with regional cervical block, the carotid shunt was inserted when the patients developed mood change, or speech or motor dysfunction. A carotid shunt was used routinely in selected patients with general anesthesia. Endarterectomy was performed in the standard fashion and patch angioplasty with bovine pericardium patch (Vascu-Guard, Bio-Vascular, Saint Paul, MN, USA) was performed in the majority of patients. The sutures were tacked to secure the distal intimal flap and ICA plication if necessary.8 Completion imaging at the time of the endarterectomy was used selectively. When distal anastomosis site narrowing or distal dissection with flow disturbance was suspected, duplex scanning or angiography was performed upon completion of the surgery.
All patients were monitored in the intensive care unit for at least 8 hours postoperatively, and were followed up both clinically (by a neurologist for patients who developed new neurological symptoms) and by magnetic resonance imaging (MRI) with angiography before discharge, followed by color-flow duplex ultrasonography at 3 months postoperatively and then every year thereafter. Patients with a neurological deficit were followed up by both a neurologist and vascular surgeon after discharge. The presence of a restenosis was evaluated in the duplex ultrasonography findings, making several measurements in different planes. The blood volume flow, velocity, and spectral analysis waveform were also measured. For this study, restenosis was defined as recurrent luminal narrowing of >50% with a peak systolic velocity of >125 cm/s at the endarterectomy site. Patient characteristics, comorbidities, stroke, mortality, restenosis, cause of death, technical aspects of the CEA (use of a shunt and a patch), long-term outcomes, and other surgical complications were obtained from a review of medical records. A major infarction with sequelae was defined as a neurological deficit lasting more than 6 months with proximal infarction in the cerebral arterial territory (distal ICA, proximal middle cerebral artery, or basilar artery) at the follow-up imaging study, and when the patient could not manage activities of daily living without assistance from others, had dysphasia, or was unable to take part in simple communication. A minor infarction was considered as a stroke only if symptoms or signs lasted longer than 24 hours and led to some lifestyle restrictions or did not interfere with the patient's lifestyle. Postoperative stroke (defined as an acute neurological event with focal symptoms and signs lasting for 24 hours or more) and death were considered major complications; other surgical complications such as cranial nerve injury, wound hematoma, wound infection, and other cardiovascular problems were defined as minor complications. The perioperative period was defined as the 30-day period after surgery, and the postoperative period as the period from the surgical procedure until either the surgeon discharged the patient from their care or was fully recovered without a time limit of 30 days after surgery. Stroke-related death was considered when there was a history of stroke and no more obvious specific cause or death from cerebrovascular disease. Stroke-related death was defined as 1) stroke-induced brain herniation or respiratory arrest from brainstem stroke, 2) aspiration caused by stroke, or 3) an immobilization-related event (e.g., sepsis from a decubitus ulcer, or pulmonary embolism from deep venous thrombosis) including those occurring in the nonacute phase after stroke. The data were collected from medical records and a call to each patient to gather information on patients who had died before July 1, 2012.
The R package (version 3.0.2, R Foundation for Statistical Computing, Vienna, Austria) and the Statistical Package for the Social Sciences (version 19.0, SPSS Inc., Chicago, IL, USA) were used for all statistical analyses. Cox multiple-regression models were used to calculate the hazard ratio (HR), 95% confidence interval (CI), and corresponding p values. Gender and diabetes were used as covariates. Differences in mortality and stroke-related mortality according to age were analyzed with aged <70 years as a reference group. The chi-square test and Fisher's exact test were used to compare risk factors and mortality among subgroups. Kaplan-Meier curves and estimates of survival data were used to account for differing survival times. The cutoff for statistical significance was set at p<0.05.
In total, 649 CEA procedures were performed in 596 patients, with 53 patients undergoing bilateral CEA. The baseline characteristics of the patients who underwent CEAs are given in Table 1. The male-to-female ratio was 7.2:1, and the mean age was 67.5 years.
CEA with patch angioplasty was the main surgical method used in this study, with an autologous vein patch being used in 137 cases, a bovine patch in 485, and a polytetrafluoroethylene patch in 4. CEA was performed with primary closure in 11 cases and with eversion in 12. With regard to anesthesia, regional cervical block and general anesthesia were performed in 546 and 103 cases, respectively. In 2 patients, regional cervical block was performed initially, but was converted to general anesthesia during surgery due to mood change. A shunt was used in 123 of the 649 CEAs: in 62 of 546 with regional anesthesia (11.4%) and in 61 of 103 with general anesthesia (59.2%). The operative details of the CEAs are given in Table 2.
Following CEA, 591 cases underwent MRI prior to discharge. There were abnormalities on MRI consistent with asymptomatic emboli. Review of the entire MRI results revealed 23 cases of asymptomatic emboli; however, there were no MRI images for 58 cases in the early study period. In practice, it is possible that asymptomatic emboli can be observed in a greater number of cases. The patency of the ipsilateral carotid artery was assessed with the aid of duplex ultrasonography during the follow-up period. Carotid restenosis after CEA occurred after a median of 6 months (range: 0-24 months). Restenosis was defined as recurrent luminal narrowing of >50% at the endarterectomy site, and this was detected in 11 cases (1.69%), all of which were asymptomatic. Use of the cumulative event rate to calculate restenosis during follow-up revealed restenosis rates of 1.1% and 1.9% at 24 months and 48 months, respectively.
Perioperative ipsilateral stroke occurred in seven cases (1.07%), of which four and three were symptomatic and asymptomatic preoperatively, respectively. Perioperative stroke was diagnosed both clinically by a neurologist for patients who developed new neurological symptoms and by MRI with angiography. A major ipsilateral infarction with sequelae occurred in three patients, and a minor ipsilateral infarction without sequelae occurred in four. Contralateral infarction occurred in 23 patients during the follow-up period. There were 2 perioperative contralateral infarctions, and 3 cases that developed during follow-up were major contralateral infarction; the remaining 18 cases were all asymptomatic minor infarction. Furthermore, there were 15 cases of cranial nerve injury, 20 of laryngeal edema, 3 of hyperperfusion syndrome, and 18 of bleeding and hematoma (2 of which required revision) (Table 3).
The median follow-up period over the 10-year study period was 48 months; 104 patients died during the follow-up period (17.4%). Within 30 days of surgery, there were two deaths and nine strokes, giving a combined rate of perioperative death or stroke of 1.84%. The cause of death was investigated to identify the relevance of CEAs and deaths, and divided into stroke-related and stroke-unrelated deaths. There were 16 stroke-related deaths, including deaths from aspiration or cerebrovascular disease, and 88 stroke-unrelated deaths, including deaths from cancer (n=31), heart problems (n=18), pneumonia (n=11), aging (n=9), and end-stage renal disease (n=4). Use of the cumulative event rate to calculate the rates of mortality and stroke-related death during follow-up yielded mortality rates of 7.6% and 16.5%, and stroke-related death rates of 0.6%, 2.8% at 24 months and 48 months, respectively.
Differences in comorbidity and mortality were analyzed according to age, and with patients stratified according to the following age groups: <70 years, 70-79 years, and ≥80 years. The proportions of symptomatic carotid artery stenosis did not differ among these three age groups [63.0% (237/376), 63.8% (157/246), and 62.9% (17/27), respectively; p=0.920]. There were also no significant differences among the three groups with regard to comorbid conditions, except for those with diabetes. The rate of diabetes was lowest in the octogenarian group. The overall mortality rate was higher among patients in the 70-79 years and ≥80 years age groups than those aged <70 years (70-79 years: HR=1.80, 95% CI=1.2-2.6, p=0.003; ≥80 years: HR=3.77, 95% CI=1.7-8.4, p=0.001); however, there were no significant differences in stroke-related mortality among the three age groups. The comorbidity and mortality rates in the three age groups are given in Table 4 and 5, and Kaplan-Meier curves illustrating overall survival and stroke-related survival are shown in Fig. 1. Differences in mortality and stroke-related mortality according to age were analyzed using patients aged <70 years as a reference group. The high-risk group of patients had a higher overall mortality than the low-risk group (HR=2.38, 95% CI=1.4-4.2, p=0.003), but there was no significant difference in stroke-related mortality between the two groups. An analysis of the mortality results for each risk group is shown in Fig. 2.
CEA has been performed in many institutions in many countries, and the outcomes identified in large randomized controlled trials (RCTs) of CEA are in broad agreement. As expected at the beginning of this study, the results presented herein are consistent with those of large international reviews. CAS has recently emerged as an alternative treatment for patients considered with an increased risk of perioperative complications. However, defining which patients actually fall into this high-risk group has been difficult, and it is unclear whether the incidence of complications after CEA is increased in elderly and high-risk patients. Patients at high risk for CEA or CAS might be difficult to randomize due to concerns regarding possible complications and/or poor outcomes. Therefore, the aim of this study was to identify whether CEA is a safe procedure in elderly and high-risk patients.
A relatively low rate of adverse events after CEA was found, with a perioperative death or stroke rate of 1.84% and a cranial nerve injury rate of 2.3%. The stroke/mortality rate is comparable to that found in both the European Carotid Surgery Trial (ECST) and the North American Symptomatic Carotid Surgery Trial (NASCET).34910
The incidence of cranial nerve injury reported in the literature ranges from 3% to 47.5%, depending on whether the study was conducted retrospectively or prospectively and on the sensitivity of the method used to investigate nerve damage.51011 The reported rates of postoperative cranial nerve injury found in both NASCET (8.6%) and ECST (5.1%) were considerably higher than in the present study (2.3%).410 Most of those injuries in the present study were temporary, with permanent facial nerve deficit noted in only one patient who had undergone revision of the wound due to postoperative bleeding. However, since the neurological evaluation for cranial nerve injuries was limited to symptomatic patients, it is possible that transient cranial nerve injuries that were clinically insignificant went undetected in this study.3
The risk factors for shunting include a reduction in the patent segment in the contralateral hemisphere or absence of both the anterior and posterior communicating arteries in the preoperative MRA.7 In patients with regional cervical blockage, the carotid shunt was inserted based on alterations that developed after the carotid artery was clamped, as found in a neurological examination. A carotid shunt was used routinely in patients under general anesthesia; however, CEA was performed without routine shunting in selected patients under general anesthesia in the later period of the study. Shunt use was determined by preoperative MRA and intraoperative cerebral oximetry; preoperative MRA is valuable when predicting cerebral ischemia during CEA. CEA was performed without a shunt if there were patent intracranial arteries in the contralateral hemisphere and patent communicating arteries, but shunting was performed if cerebral oximetry fell by >10% during carotid artery clamping. Perioperative stroke or death did not occur in nonshunted patients in this study. In principle, we use carotid shunt routinely in patients with general anesthesia.
There were 16 stroke-related deaths during the 10-year period, with the remaining deaths being due to other causes. Comorbidities and life expectancy appear to have a considerable impact on mortality, since cancer and heart disease accounted for almost half of the deaths in this study (not including patients with an unknown cause of death).
A subgroup analysis of the pooled data from large RCTs revealed that men and patients aged >75 years received the greatest benefit from CEA.5 Although poor health may be a reason for denying elderly patients access to CEA, the NASCET results for 409 elderly patients showed that for all degrees of stenosis, medically treated elderly patients had the highest risk of stroke. Therefore, elderly patients without organ failure or serious cardiac dysfunction are ideal candidates for CEA.12
Several studies have found that being older than 80 years as a univariate risk factor of death or stroke, but interpreting the findings is limited by a lack of formal multivariable analyses in most cases. In addition, most of the RCTs excluded patients aged >80 years-as well as those with major comorbidities-due to concerns regarding a higher risk and limited life expectancy. RCTs comparing CEA and CAS have found a considerably higher risk of perioperative complications in patients aged ≥80 years. Together, these findings suggest that octogenarians comprise a high-risk group for whom the benefits of any carotid revascularization (CEA or CAS) may be greatly diminished compared with their younger counterparts.13 However, in the present study there were no significant differences in comorbidity among the three age groups, except for those with diabetes. There were fewer diabetic patients in the octogenarian group, which may be attributed to diabetic patients dying before the age of 80 years due to diabetic complications.
While the overall mortality rate was higher among patients in the 70-79 years and ≥80 years age groups than in the <70 years age group, there were no differences between the groups with regard to stroke-related mortality. Therefore, CEA may be performed in elderly patients with acceptable life expectancy.
We suggest that CEA can be performed safely in elderly patients previously considered as "high risk" and should remain the standard of care. Although the higher overall mortality in high-risk patients may be attributed to their existing conditions, the stroke-related mortality did not differ between the two groups.
The findings of this study should be considered in the light of several limitations, primarily related to its retrospective design. First, selection bias may have been present in deciding when to operate on the patients. However, the possibility of selection bias was minimized because all surgeries were performed by three surgeons and relatively few complications occurred despite the high yearly volumes, and all of the patients were assessed preoperatively by a neurologist.14 Second, relatively few octogenarians and high-risk patients were included in this study. More large-scale studies are needed to further investigate the outcome of CEA in elderly and high-risk patients. Third, CAS was conducted in 532 patients between 2001 and 2010 at our institution. It is possible that high-risk patients underwent CAS whereas low-risk patients underwent CEA, and that the safety and effectiveness of CEA may have been overestimated. Fourth, no follow-up duplex ultrasonography scan was performed in 41 cases. MRA images were obtained in patients who did not undergo carotid duplex ultrasonography after discharge. Although there was no evidence of restenosis on MRA, this is not a precise test for detecting restenosis, and so the restenosis rate may have been underestimated.
In conclusion, evaluation of the outcomes of CEA over a 10-year period has yielded acceptable results in terms of stroke and mortality, with findings that are in agreement with those of large international trials. Although patients in the 70-79 years and ≥80 years age groups had a higher overall mortality than those in the <70 years age group, there were no differences between the groups with regard to stroke-related mortality. The recent increase in the average life expectancy and improvements in quality of life are expected to result in an increased number of elderly patients. Given that CEA is a safe and effective strategy, it may be performed in elderly patients with acceptable life expectancy. Future large-scale studies are needed to further investigate the outcomes of CEA in elderly and high-risk groups.
Figures and Tables
Fig. 1
Survival in the patients stratified according to age as follows: 70 years, 70-79 years, and ≥80 years. Kaplan-Meier curve illustrating the overall survival relative to age. Patients aged 70-79 years and ≥80 years had a higher overall mortality rate than those aged <70 years. B: Kaplan-Meier curve illustrating stroke-related survival relative to age. There was no significant difference in stroke-related mortality among the three age groups studied.

Fig. 2
A: Kaplan-Meier curve illustrating overall survival in the patients stratified according to surgical risk: high and low. The high-risk group had a higher overall mortality than the low-risk group. B: Kaplan-Meier curve illustrating stroke-related survival in the two surgical risk groups. There was no significant difference in stroke-related mortality between the two groups.

Table 1
Baseline characteristics of patients who underwent carotid endarterectomy (CEA)
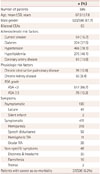
Table 2
Operative details of the carotid endarterectomy (CEA) procedures

Table 3
Perioperative complications within 30 days of carotid endarterectomy

Table 4
Analysis of comorbidity and mortality in the 3 age groups

Table 5
Comparison of overall mortality and stroke-related mortality for each age group

References
1. Hong KS, Bang OY, Kang DW, Yu KH, Bae HJ, Lee JS, et al. Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the Korean Stroke Society and clinical research center for stroke. J Stroke. 2013; 15:2–20.

2. Eastcott HH, Pickering GW, Rob CG. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet. 1954; 267:994–996.

3. Duncan JM, Reul GJ, Ott DA, Kincade RC, Davis JW. Outcomes and risk factors in 1,609 carotid endarterectomies. Tex Heart Inst J. 2008; 35:104–110.
4. Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999; 30:1751–1758.

5. Lovrencic-Huzjan A, Rundek T, Katsnelson M. Recommendations for management of patients with carotid stenosis. Stroke Res Treat. 2012; 2012:175869.

6. Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Stroke. 2011; 42:e420–e463.
7. Shin S, Kwon TW, Cho YP, Shin JH, Yi A, Kim H, et al. Preoperative magnetic resonance angiography as a predictive test for cerebral ischemia during carotid endarterectomy. World J Surg. 2013; 37:663–670.

8. Kim JH, Cho YP, Kwon TW, Kim H, Kim GE. Ten-year comparative analysis of bovine pericardium and autogenous vein for patch angioplasty in patients undergoing carotid endarterectomy. Ann Vasc Surg. 2012; 26:353–358.

9. Dahl T, Aasland J, Romundstad P, Johnsen HJ, Myhre HO. Carotid endarterectomy: time-trends and results during a 20-year period. Int Angiol. 2006; 25:241–248.
10. Cunningham EJ, Bond R, Mayberg MR, Warlow CP, Rothwell PM. Risk of persistent cranial nerve injury after carotid endarterectomy. J Neurosurg. 2004; 101:445–448.

11. Ballotta E, Da Giau G, Renon L, Narne S, Saladini M, Abbruzzese E, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999; 125:85–91.

12. Barnett HJ, Meldrum HE, Eliasziw M. North American Symptomatic Carotid Endarterectomy Trial (NASCET) collaborators. The appropriate use of carotid endarterectomy. CMAJ. 2002; 166:1169–1179.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download