Abstract
Background and Purpose
Transient global amnesia (TGA) is a stereotypic condition characterized by anterograde and retrograde amnesia that typically resolves within 24 hours. The pathophysiology of TGA is still unclear. We noted that patients hospitalized with TGA tend to appear in seasonal clusters, and decided to investigate this phenomenon.
Methods
Every patient with acute presentation of amnesia at our medical center is hospitalized for observation and evaluation. We reviewed the monthly occurrence of TGA in our patient population between 2000 and 2014, and compared this to non-TGA hospitalizations during the same time period.
Results
During the analysis period, 154 patients who met the criteria for TGA were hospitalized, as well as 259,007 non-TGA hospitalizations. The annual occurrence of TGA ranged from 5 to 16 hospitalizations. There were 91 TGA events in women and 63 in men, in subjects aged 62.8±10.6 years (mean±SD). The incidence was maximal during December [odds ratio (OR)=2.83, 95% confidence interval (CI)=1.20–6.67] and March (OR=2.77, 95% CI=1.17–6.56), and minimal from April to August. The incidence exhibited an increase followed by a decrease from October to February. A seasonal trend was observed as well, with incidence peaks occurring in winter (OR=1.82, 95% CI=1.12–2.96) and spring (OR=1.80, 95% CI=1.10–2.94).
Transient global amnesia (TGA) is an intriguing clinical condition hallmarked by the sudden onset of deficit in anterograde memory that usually resolves over a day.12 It is thought to be benign, although slight memory deficits might persist for months after an episode.3 Case series in which diffusion-weighted imaging (DWI) was performed on TGA patients revealed focal areas of restricted diffusion, confined to the CA1 area of the hippocampus.4 The incidence of TGA is approximately 20 per 100,000 persons among those aged 50–80 years.5
The exact cause of TGA remains elusive.6 There is evidence that TGA is correlated with transient ischemic attacks (TIAs),78 venous congestion,910 and a migrainous phenomenon,11 but definitive proof supporting any of these mechanisms is lacking.12
Every patient with an acute presentation of isolated amnesia in our medical center is admitted for further observation and evaluation. We noted that patients hospitalized with TGA tend to appear in clusters. Moreover, we noted that most admissions with TGA to the neurology department occurred during winter and spring. We therefore decided to analyze whether this phenomenon is consistent for admissions in the internal medicine wing of the entire hospital over several years. Our initial review confirmed that this observation is indeed valid and encouraged us to further assess the epidemiology of TGA admissions over a 15-year period.
Discharge data were collected retrospectively from a single tertiary care center in Israel over a period of 15 years, between January 2000 and December 2014 (Table 1). Data collection was approved by the Institutional Review Board and authorized for patient anonymous inclusion. For each patient we procured the date of hospitalization, diagnosis given at discharge, age, and sex. Data were divided according to the discharge diagnosis into TGA hospitalizations and non-TGA hospitalizations in the internal medicine wing (which includes the neurology ward).
TGA was diagnosed using the following accepted criteria:13 1) presence of an anterograde amnesia event that is witnessed by an observer, 2) the cognitive impairment is limited to amnesia, 3) no focal neurological signs, 4) no recent history of head trauma or seizures, and 5) resolution of symptoms within 24 hours. All patients underwent a neurological examination upon admission, noncontrast head CT and inpatient follow-up for at least 24 hours in order to exclude TGA-mimicking conditions.
We plotted the incidence of TGA admissions for each month (Fig. 1) and season (Fig. 2) over the analyzed 15-year period.
Our statistical hypotheses were that the incidence of TGA varies with the month or season. For this analysis, hospitalization data were grouped into seasons defined according to the Gregorian calendar.
Statistical methods for the assessment and quantification of seasonality were chosen based on an extensive review14 and our previous experience. The methods considered range from a comparison of disease incidence (percentage of TGA hospitalizations relative to the total number of hospitalizations) between the extremes of summer and winter, through modeling of the intensity of seasonal patterns by use of a periodogram, to more-advanced generalized linear models. Since the outcome variable was binary, a logistic regression was fitted to the data, with month or season as a categorical dependent variable. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated as estimates of relative risk. Summer was used as the reference category for the seasonal analysis, while the month of June was used for the monthly analysis.
We have also performed a time series analysis through a spectral density, and presented the data as a periodogram, since such plots may be used to identify dominant periods of time series. We used Fourier transformation implemented using SPSS software to calculate the intensity.
During this 15 year period, 259,161 hospital admissions occurred, of which 173 had been diagnosed as being due to transient acute isolated amnesia. Of these, 19 were excluded because they were eventually diagnosed as epilepsy or another condition. The remaining 154 patients met the criteria for TGA (Table 1). There were 91 TGA events in women and 63 in men (with 4 recurrent episodes, 2.5%), in subjects aged 62.8±10.6 years (mean±SD).
The monthly incidence of TGA peaked twice within each year (Figs. 1 and 2, Table 2 and 3). The first peak occurred during December (OR=2.83, 95% CI=1.20–6.67), with the second occurring during March (OR=2.77, 95% CI=1.17–6.56). The incidence decreased during April through August, during which there were no significant variations in incidence. The incidence exhibited an increase followed up by a decrease from October to February, peaking in December.
We have retrospectively analyzed seasonal patterns in the incidence of TGA at a tertiary medical center over a 15-year period. Our study investigated a relatively large number of subjects over a long duration. We have observed that the incidence of TGA exhibits both monthly and seasonal peaks, with these results being confirmed via logistic regression and time-season analysis. This observation may help to improve the understanding of the pathophysiology underlying this condition.
Significant alterations in physiological parameters have been previously noted to alternate during the seasons, including hormonal changes, production of coagulation factors, oxidative stress, endothelial dysfunction, mild changes in hemodynamic parameters, immunological changes, and susceptibility to infectious agents.15161718 These alterations are thought to follow meteorological seasonal changes such as air pollution, UV radiation, temperature, and atmospheric air pressure. Lifestyle activity and dietary habits exhibit normal fluctuations with additional background health impacts. Both vascular and infectious clinical phenomena follow seasonal trends. Venous and arterial thrombosis have a greater tendency to occur during the winter months,15 including TIA and ischemic strokes. One previously unconsidered possibility is that TGA is triggered by an infection, or is part of a postinfectious phenomenon.192021 Previous studies have observed DWI restriction on magnetic resonance imaging (MRI) in TGA patients. These lesions were bilateral and symmetric,5 similar symmetric MRI lesions were also seen in central-nervous-system viral infections, such as herpes simplex encephalitis. Infections tend to exhibit seasonal variations. Examples include enteroviruses that peak during summer and autumn, and the measles virus that may exhibit an outbreak in the autumn. Examples of viruses that have peaks of infection in early spring and winter are respiratory syncytial virus20 and rotavirus.22 However, additional research is needed to support the speculation that infection is a possible etiology of TGA.
Akkawi et al.23 described an association between TGA occurrence and low ambient temperature. The age, sex ratio, and annual distribution of TGA in our patient population are consistent with data reported by that group. That study reviewed all TGA cases admitted to an Italian hospital over a 6-year period. The study did not analyze seasonal or monthly variability, but consisted of a comparison between the climatic parameters of days with and without TGA. Most of the TGA cases occurred when the outdoor temperature was less than 6.9℃, with the frequency of TGA being a minimum when it exceeded 24℃ (p<0.0001). Since lower temperatures are observed in Israel and Italy during the winter and early spring, this data supports the findings of our study.
Our study may have underrepresented cases whose care was managed in the community. However, the clinical phenotype of TGA is usually alarming, and patients in Israel are generally referred to hospitals for further medical evaluations. Due to the proximity to major hospitals in the center of Israel, nearly all cases of acute confusion states received emergency-room attention within 24 hours of onset.
We also observed some year-to-year variability. Our tertiary medical center and five other medical centers service the bulk of the center of Israel (approximately 3 million people). We expected some degree of year-to-year variability due to an unequal distribution of cases between these medical centers.
In conclusion, our findings suggest that TGA has seasonal incidence variations, which supports the findings of Akkawi et al.23 Since this observation is supported by evidence from a previous study performed in another Mediterranean country, we believe that further comparison of TGA epidemiology in various countries will yield new insights and may facilitate a better understanding of its pathophysiology.
Figures and Tables
Fig. 1
Incidence rate of TGA per month (numbers are in the scale of 10-6). TGA: transient global amnesia.

Fig. 2
Incidence rate of TGA per season (numbers are in the scale of 10-6). TGA: transient global amnesia.

Table 1
Annual incidence rates of TGA during 2000–2014: incidence, occurrence, sex, and age
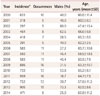
Table 2
OR and 95% CI values for the incidence of TGA according to month
References
2. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010; 9:205–214.

3. Noël A, Quinette P, Dayan J, Guillery-Girard B, Piolino P, Pélerin A, et al. Influence of patients' emotional state on the recovery processes after a transient global amnesia. Cortex. 2011; 47:981–991.

4. Bartsch T, Alfke K, Stingele R, Rohr A, Freitag-Wolf S, Jansen O, et al. Selective affection of hippocampal CA-1 neurons in patients with transient global amnesia without long-term sequelae. Brain. 2006; 129:2874–2884.

5. Koski KJ, Marttila RJ. Transient global amnesia: incidence in an urban population. Acta Neurol Scand. 1990; 81:358–360.

7. Zorzon M, Antonutti L, Masè G, Biasutti E, Vitrani B, Cazzato G. Transient global amnesia and transient ischemic attack. Natural history, vascular risk factors, and associated conditions. Stroke. 1995; 26:1536–1542.

8. Jang JW, Park SY, Hong JH, Park YH, Kim JE, Kim S. Different risk factor profiles between transient global amnesia and transient ischemic attack: a large case-control study. Eur Neurol. 2014; 71:19–24.

9. Akkawi NM, Agosti C, Rozzini L, Anzola GP, Padovani A. Transient global amnesia and venous flow patterns. Lancet. 2001; 357:639.

10. Sander D, Winbeck K, Etgen T, Knapp R, Klingelhöfer J, Conrad B. Disturbance of venous flow patterns in patients with transient global amnesia. Lancet. 2000; 356:1982–1984.

11. Schmidtke K, Ehmsen L. Transient global amnesia and migraine. A case control study. Eur Neurol. 1998; 40:9–14.
12. Tong DC, Grossman M. What causes transient global amnesia? New insights from DWI. Neurology. 2004; 62:2154–2155.

13. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990; 53:834–843.

14. Christiansen CF, Pedersen L, Sørensen HT, Rothman KJ. Methods to assess seasonal effects in epidemiological studies of infectious diseases--exemplified by application to the occurrence of meningococcal disease. Clin Microbiol Infect. 2012; 18:963–969.

16. Radke KJ, Izzo JL Jr. Seasonal variation in haemodynamics and blood pressure-regulating hormones. J Hum Hypertens. 2010; 24:410–416.

17. Stout RW, Crawford V. Seasonal variations in fibrinogen concentrations among elderly people. Lancet. 1991; 338:9–13.

18. Shahar DR, Froom P, Harari G, Yerushalmi N, Lubin F, Kristal-Boneh E. Changes in dietary intake account for seasonal changes in cardiovascular disease risk factors. Eur J Clin Nutr. 1999; 53:395–400.

19. Lopez J, Lomen-Hoerth C, Deutsch GK, Kerchner GA, Koshy A. Influenza-associated global amnesia and hippocampal imaging abnormality. Neurocase. 2014; 20:446–451.

20. Fisman D. Seasonality of viral infections: mechanisms and unknowns. Clin Microbiol Infect. 2012; 18:946–954.

21. Rösler A, Mras GJ, Frese A, Albert I, Schnorpfeil F. Precipitating factors of transient global amnesia. J Neurol. 1999; 246:53–54.

22. Bruijning-Verhagen P, Quach C, Bonten M. Nosocomial rotavirus infections: a meta-analysis. Pediatrics. 2012; 129:e1011–e1019.

23. Akkawi NM, Agosti C, Grassi M, Borroni B, Pezzini A, Vignolo LA, et al. Weather conditions and transient global amnesia. A six-year study. J Neurol. 2006; 253:194–198.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download