Abstract
No disease-modifying therapies (DMT) for neurodegenerative diseases (NDs) have been established, particularly for Alzheimer's disease (AD) and Parkinson's disease (PD). It is unclear why candidate drugs that successfully demonstrate therapeutic effects in animal models fail to show disease-modifying effects in clinical trials. To overcome this hurdle, patients with homogeneous pathologies should be detected as early as possible. The early detection of AD patients using sufficiently tested biomarkers could demonstrate the potential usefulness of combining biomarkers with clinical measures as a diagnostic tool. Cerebrospinal fluid (CSF) biomarkers for NDs are being incorporated in clinical trials designed with the aim of detecting patients earlier, evaluating target engagement, collecting homogeneous patients, facilitating prevention trials, and testing the potential of surrogate markers relative to clinical measures. In this review we summarize the latest information on CSF biomarkers in NDs, particularly AD and PD, and their use in clinical trials. The large number of issues related to CSF biomarker measurements and applications has resulted in relatively few clinical trials on CSF biomarkers being conducted. However, the available CSF biomarker data obtained in clinical trials support the advantages of incorporating CSF biomarkers in clinical trials, even though the data have mostly been obtained in AD trials. We describe the current issues with and ongoing efforts for the use of CSF biomarkers in clinical trials and the plans to harness CSF biomarkers for the development of DMT and clinical routines. This effort requires nationwide, global, and multidisciplinary efforts in academia, industry, and regulatory agencies to facilitate a new era.
Alzheimer's disease (AD) is a major cause of dementia and the most common neurodegenerative disease (ND),12 while Parkinson's disease (PD) is the second most common form of ND and is associated with progressive motor dysfunction.3 Many NDs, particularly AD and PD, are irreversible and progressive, and they severely affect the quality of life of the patients and their caregivers as well as result in large socioeconomic costs. Therefore, the early diagnosis of NDs, the identification of people with risk factors, and the development of disease-modifying therapies (DMT) are critical to decreasing the socioeconomic burden of NDs. However, the current approaches are not satisfactory for many reasons, including since the diagnosis of ND is conducted using clinical diagnostic criteria (e.g., AD diagnosis by NINCDS-ADRDA criteria) that have a low diagnostic performance.45 Furthermore, it is widely accepted that the neuropathogenic changes of AD (i.e., amyloid beta deposition and tau pathology) occur at least 20 years before symptom onset.678 The typical clinical motor symptoms in PD (i.e., tremor, bradykinesia, and rigidity) are not detected before at least 50% of the dopaminergic neurons in the pars compacta of the substantia nigra have died, with this masking effect being due to presymptomatic compensation.9 The diagnostic accuracy of advanced PD in specialized centers can be 90%,10 but it can be much lower in nonspecialized settings and/or in earlier stages. Moreover, the clinical symptoms and even neuropathologies frequently overlap among the NDs (e.g., dementia with Lewy bodies, AD, and PD) and with non-NDs (e.g., PD and essential tremor), thus leading to misdiagnosis. It is therefore necessary to develop valid biomarkers to increase the diagnostic accuracy of clinical diagnosis of NDs, replace clinical diagnostic criteria, and/or predict future disease progression.
A biomarker is defined as a characteristic that is objectively measured and evaluated as an indicator of a normal biological process, pathogenic process, or pharmacological response to a therapeutic intervention,11 and this includes imaging, biochemical, and genetic biomarkers. In NDs, biomarkers that are derived from the cerebrospinal fluid (CSF), which reflects molecular events in the brain due to it being in direct contact with the extracellular spaces,12 have been most intensely studied. Numerous clinical studies involving the development of valid biomarkers for ND diagnosis, particularly of AD, have been conducted, as well as clinical trials including CSF biomarkers that aimed to gather study participants, engage therapeutic targets, monitor pharmacodynamic effects, and/or predict disease progression. In this review we briefly discuss the clinical utility of CSF biomarkers for NDs, particularly AD and PD. In addition, we describe the potential roles of CSF biomarkers in the process of drug development, including clinical trials, and the challenges in the use of CSF biomarkers.
Various NDs are caused by protein misfolding and aggregation, and the commonality of these molecular mechanisms suggests that proteinopathy is involved. For example, a major hypothesis of AD development is that the accumulation and aggregation of amyloid beta1–42 (Aβ42) induces progressive synaptic and neuronal injury and activates microglia and astrocytes, followed by oxidative stress, widespread neurite dysfunction, and neuronal death. PD is also a proteinopathy, and is caused by the misfolding of proteins including alpha-synuclein (α-syn). These pathogenic proteins accumulate due to imbalance between the production and clearance systems resulting from genetic and/or nongenetic factors. Impairment of the ubiquitin-proteasome system is an example outcome of the failure to clear pathogenic proteins, followed by the formation, aggregation, and deposition of toxic oligomers intracellularly or extracellularly.1314 These pathogenic mechanisms of proteinopathies might therefore be suitable targets of DMT and for the development of biomarkers. Previous studies of CSF biomarkers of NDs have focused on proteins involved in disease-specific pathogenic processes, although NDs have clinical and/or pathological heterogeneities, and overlapping symptoms or neuropathologies. Based on these elucidated pathogenic mechanisms, numerous candidate DMT that target the pathogenic mechanisms are currently being developed. Fig. 1 illustrates the sequences of proteinopathic mechanisms (protein misfolding → aggregation → intracellular or extracellular deposition) of AD and PD, the accompanying therapeutic targets, and candidate drugs.
Numerous forms of Aβ are found in amyloid plaque (AP) and are spliced from membrane-spanning amyloid precursor protein (APP) by α-, β-, and γ-secretases. Among them, Aβ42, which is the stickiest fragment, is produced by β- and γ-secretases.15 The overproduction of Aβ42 and/or impairments in Aβ42 clearance induce(s) the accumulation of Aβ42, production of toxic oligomeric Aβ, and aggregation and deposition in the AP,16,17,18 and these mechanisms are related to the low concentrations of Aβ42 measured in the CSF of patients with AD.1920 Neurofibrillary tangles (NFTs) consist of aggregations of the hyperphosphorylated form of the microtubule-associated protein tau that is assembled into paired helical filaments, which causes a loss of microtubulestabilizing functions. Many kinases and phosphatases regulate the levels of tau phosphorylation at multiple serine and threonine residues. Phosphorylated tau (p-tau) is hypothesized to be induced by Aβ toxicity, and increased levels of total tau (t-tau) and p-tau in the CSF are thought to reflect the release of tau-related protein into the extracellular CSF compartment resulting from neuronal death.21 The total amount of tau in the CSF, which probably reflects the degree of neurodegeneration, is therefore called a state marker rather than a stage marker; an example of the latter is hippocampal atrophy, which indicates how far the neurodegeneration has proceeded.2223 However, the sequential relationship between Aβ accumulation, tau phosphorylation, and neuronal death has not been fully characterized. Together these findings indicate that the pharmacodynamic effects of drugs targeting Aβ deposition or tau phosphorylation may be assessed by measuring the alterations in the levels of CSF of Aβ, t-tau, and p-tau, even though the relationship between the therapy-induced alterations in these CSF biomarkers and the clinical outcome is still unknown.
The CSF concentration of Aβ42 is typically lower while those of t-tau and p-tau are typically higher in patients with AD than in healthy elderly subjects.242526 These CSF findings are typically found decades before clinically detectable cognitive dysfunction, accompanying imaging abnormalities [e.g., detection of amyloid deposition by positron-emission tomography (PET)], and even before abnormalities are detected in imaging biomarkers (e.g., hippocampal atrophy or low glucose metabolism).27 Using the core biomarkers that reflect the combination of low CSF Aβ levels and high CSF t-tau concentrations, patients with AD can be successfully differentiated from healthy elderly subjects with high (>85%) sensitivity and specificity.28 In addition, combining the core CSF biomarkers with a genetic risk factor (ApoE genotypes; ε4 positive vs. negative) can be used to predict the progression of amnestic mild cognitive impairment (MCI) to AD.29
However, several challenges restrict the use of core biomarkers as routine clinical laboratory measurements. One is the small amount of qualified data on the measurements of biomarkers, including, but not limited to, probable preanalytical and analytical variability sources of immunoassay-based quantification that influence the measured values. To overcome these challenges, members of the Alzheimer's Biomarkers Standardization Initiative recently performed a standardization study of minimizing sources of preanalytical variability.30 Immunoassay-based platforms measure CSF concentrations of biomarkers as relative levels of proteins rather than as absolute concentrations, and so they are probably influenced by several factors, including matrix effects. To this end, the Global Consortium for the Standardization of CSF Biomarkers, which is supported by the Alzheimer's Association, set out to develop internationally available certified reference materials and methods in collaboration with the Institute for Reference Materials and Measurements and the International Federation of Clinical Chemistry. Such multidisciplinary efforts are likely to facilitate the use of CSF biomarkers in clinical routines. In addition to the core CSF biomarkers, several novel biomarkers that reflect AD pathophysiological processes have also been suggested. For example, β-secretase activity in the CSF and the levels of APP fragments [soluble amyloid precursor protein alpha and beta (sAPPα and sAPPβ)], Aβ oligomers, and C-terminal-truncated Aβ species (e.g., Aβ37, Aβ38, Aβ39, Aβ14, Aβ15, and Aβ16) will soon be tested in clinical trials.31 Moreover, several studies that have measured these additional candidate novel biomarkers for AD used CSF samples from relatively small cohorts of patients whose diagnoses were determined clinically and often found to be inaccurate. Therefore, cross-sectional studies that employ clinical diagnoses should be large enough to compensate for this issue. The combination of emerging methods to image NFTs in vivo3233 and amyloid PET imaging biomarkers will be very informative in providing pathological validations of these CSF biomarkers.
α-Syn is a major component of Lewy bodies and one of the most-studied pathogenic proteins in PD.34 Physiologically α-syn is a presynaptic protein, but its functions remain to be fully elucidated. Genetic (e.g., multiplication or mutation in the SNCA gene) or nongenetic factors (e.g., posttranslational modification) produce α-syn aggregates that are toxic to mitochondria and other cellular components.35 Previous studies involving large cohorts found that the levels of α-syn in the CSF were lower in patients with PD than in controls.3637383940 However, using only CSF α-syn is not very helpful for early diagnoses of PD. A particularly interesting finding is that the levels of t-tau and p-tau in the CSF are lower in patients with PD than in controls (but not patients with AD), and the CSF α-syn levels are significantly correlated with the levels of t-tau and p-tau in the CSF in both healthy controls and patients with PD.39 The Parkinson's Progression Markers Initiative (PPMI) study is a 5-year longitudinal observational multicenter study that is evaluating the usefulness of CSF biomarkers—including core AD biomarkers and α-syn—in predictions of PD progression.3941 When combined with other CSF biomarkers, such as DJ-1, fractalkine, and AD biomarkers, α-syn may be a useful marker for predicting the progression of PD and/or reflecting the disease severity.4042 DJ-1, which is also known as PARK7, is a redox-sensitive chaperone that senses oxidative stress. Recent large cohort studies have found that the levels of DJ-1 in the CSF are lower in patients with PD than in controls, but the levels were still not useful for making accurate diagnoses. It should be kept in mind that contamination of the CSF with blood (e.g., due to traumatic lumbar puncture) increases the levels of α-syn and DJ-1; CSF hemoglobin levels are therefore measured in order to exclude contaminated samples. Standardized centrifugation of CSF immediately after performing the lumbar puncture to exclude blood cells from the CSF may help to prevent bias from preanalytical factors.394243
Core AD biomarkers have recently been applied in the early diagnosis and prediction of disease progression of patients with PD. With large degrees of heterogeneity, cognitive impairments—which are a common nonmotor morbidity— progress to overt dementia in approximately 80% of PD patients. Rapid cognitive decline in patients with PD is clearly associated with increased costs of care and higher mortality rates,4445 and its detection is critical for effective clinical management. Siderowf et al.46 reported that the 2-year cognitive decline was more rapid in PD patients with lower levels of Aβ42, which was also supported by another study.47 Therefore, the measurement of other biomarkers such as synucleinopathy- related biomarkers might be important for predicting disease progression. Similarly, clinical studies involving large cohorts and including drug-naïve patients with PD, such as the PPMI study, will be warranted for the development of valid biomarkers of PD progression. In addition, based on the experiences with AD biomarkers, the development of qualified PD biomarkers will facilitate the development of DMT for PD.
CSF biomarkers are starting to be included in clinical trials designed to develop DMT for NDs. However, the use of CSF biomarkers in clinical trials has been limited to anti-AD drugs (Fig. 2). Based on the experiences of such trials, we can describe several essential roles of CSF biomarkers to ensure the efficiency of clinical trials. These roles may be applied to other NDs including PD and other types of dementia, but it is not widely accepted to include CSF biomarker(s) in clinical trials for other NDs due to the lack of valid biomarkers (Table 1).
The currently marketed drugs for NDs are aimed at improving the clinical symptoms by targeting neurotransmitter neuronal circuits, rather than modifying the underlying pathogenic mechanisms. For example, the pharmacodynamic targets of acetylcholinesterase inhibitors and NMDA receptor antagonists in AD, and dopaminergic agonists and antimuscarinic agents in PD are not related to the pathogenic proteinopathies of these NDs. Pharmacological DMT have not been developed for AD and PD. Based on the current understanding of the pathophysiology, the discovery of novel therapeutic targets followed by their testing in preclinical studies to demonstrate their safety and effectiveness is required in animal models. The effectiveness or safety of the developed drugs is classically measured in clinical trials of phases 1 to 3, which results in a hugely expensive and lengthy development process. Reducing the failure rate and developmental period are critical to reducing the cost of new drug development. In the development of DMT in patients with NDs, the clinical effectiveness of candidate drugs needs to be determined over many years. Therefore, tools that predict the pharmacodynamic effects on a novel therapeutic target (e.g., phase-0 proof-of-mechanism study) or even the surrogate clinical efficacy will provide huge advantages and facilitate drug development.
The pharmaceutical industry uses several methods to increase the probability of the successful development of a drug, including utilizing drug-specific biomarkers, making precise measurements of the levels of target engagement, and identifying those patients who are likely to receive benefits as early as possible. As described above, biomarkers that reflect the pharmacodynamic effects are becoming increasingly valuable tools for decision-making in the development process and for the prioritization of lead compounds during preclinical and clinical studies. The aforementioned property of CSF biomarkers that reflect molecular events in the brain can be used to evaluate the levels of the intended molecular effects on the target proteins and predict the pharmacodynamic effects of DMT. This has been the main purpose of previous clinical trials designed to include CSF biomarkers. For example, drugs developed for the AD target of Aβ- producing enzymes, such as β- or γ-secretase, are expected to change the levels of proteins for APP metabolism, including Aβ and the soluble APP species.48
The administration of a β-secretase inhibitor, verubecestat (MK-8931), resulted in dose-dependent decreases in the CSF levels of Aβ40, Aβ42, and sAPPβ in a phase-1 trial,4950 and phase-2 and phase-2/3 studies are ongoing in patients with MCI and mild-to-moderate AD. A phase-2/3 trial of another β-secretase inhibitor, AZD3293 (LY3314814), is currently testing patients with MCI and mild AD following the phase-1 finding of decreased Aβ40 and Aβ42 levels and increased sAPPβ and sAPPα levels in the CSF.5152 However, evidence of efficacy on the CSF findings for target engagement not always guarantee good clinical efficacy, because safety is another key aspect of a successful drug. γ-Secretase is the target of two drugs under development, semagacestat (LY450139) and avagacestat (BMS-708163), which have shown poor clinical efficacy and show different biomarker profiles. Semagacestat did not result in a significant difference in the CSF levels of Aβ40 and Aβ42 in a phase-3 trial of patients compared with the placebo group, whereas the drug aggravated cognitive function and exhibited significant toxicity, which might have been due to the inhibition of Notch signaling.53545556 Moreover, adverse dose-limiting effects and poor tolerability of avagacestat in patients with prodromal AD who were defined by CSF biomarkers also impede further drug development. Nevertheless, treatment with avagacestat yielded evidence of dose-dependent decreases in the CSF levels of Aβ38, Aβ40, and Aβ42 in two phase-1 and -2 trials.575859 Although the avagacestat trials failed to demonstrate clinical efficacy, the findings provided important validation for the prodromal stages of AD that were defined by CSF biomarkers.
Phase-1 trials of α-syn immunotherapy (BIIB054 and PD01A) for PD are ongoing (identifiers at www.clinicaltrials.gov: NCT02459886 and NCT01568099, respectively). A phase-1 trial of a selective c-Abl kinase inhibitor (nilotinib) that enhances α-syn clearance was recently completed, and the CSF α-syn levels were included in this trial as a secondary outoutcome (NCT02281474). These results together indicate that although CSF biomarkers are not currently fully incorporated in the designs of clinical trials for DMT, the use of CSF biomarkers as secondary tools for predicting clinical efficacy will provide more information in evaluations of molecular target engagement of pharmacodynamics in clinical situations.
The clinical-diagnosis-based enrollment of patients in phase-2 and -3 trials, which is the current protocol, is unlikely to result in the recruitment of subjects with homogeneous pathological characteristics. To develop DMT targeting pathogenic components, diagnoses that are based on clinical criteria should be assisted by another tool that can stratify patients according to their pathological findings. This approach is likely to reduce the burden of excessive sample sizes that are employed to address the high degree of heterogeneity in study populations. If valid biomarkers that reflect the pathological characteristics of patients are developed, they can be used to filter study patients in the screening process, and they will result in a particular statistical power being possible using a smaller sample.60 In addition, the slow progression of most NDs can make it difficult to clinically diagnose patients in the very early stage, when the clinical symptoms are not fully exhibited. Therefore, biomarkers will allow for the design of appropriate clinical trials in the early stages of the disease with sufficient statistical power achieved by enrolling homogeneous subjects. For example, core AD CSF biomarkers show sufficient predictive values for the progression of MCI to AD, which indicates the possibility of stratifying between patients with MCI with homogeneous AD pathologies in their brains who will progress to AD and patients with MCI without AD pathology.61 This patient stratification strategy can also be applied in AD prevention trials; this issue is discussed in more detail in the next session.
The phase-1 first-in-human trial of GSK933776 (Aβ immunotherapy) only included patients with prodromal or mild AD with AD CSF profiles (low CSF Aβ42 and high t-tau or p-tau).62 In a phase-2 trial of avagacestat, the cognitive decline and brain atrophy were more rapid in patients with prodromal or mild AD with AD CSF profiles than in patients without AD CSF profiles, which indicated that utilizing CSF biomarkers can be a useful sample-enrichment strategy in clinical trials.59 Several ongoing clinical trials have applying biomarker-based inclusion criteria. The EXPEDITION 1 and EXPEDITION 2 phase-3 clinical trials that targeted soluble Aβ found clinical efficacy in the solanezumab treatment group, with the inhibition of cognitive decline [e.g., Alzheimer's Disease Assessment Scale-cognition (ADAS-cog) and Mini Mental Status Examination scores] in patients with mild AD but not those with moderate AD,6364 which suggested that CSF biomarkers are useful for showing target engagement and sample enrichment. Further trials involving patients with mild AD who are selected based on imaging and CSF biomarkers (EXPEDITION 3) are ongoing (NCT01900665). Another phase-2/3 trial of a BACE inhibitor [verubecestat (NCT01953601)] that used the CSF tau/Aβ42 ratio as the inclusion criterion is also ongoing. Together these results indicate that CSF biomarkers reflect pathological molecular changes in the brain and that their use is cost-effective. CSF biomarkers can also be useful for selecting healthy elderly subjects without AD pathology for the early phases of a clinical trial, such as in a phase-1 first-in-human study.
Because the neuronal degeneration in AD is irreversible, it is expected that the earlier treatment of a disease by DMT will result in better clinical efficacy. All efforts to develop DMT for patients with mild-to-moderate AD have failed, which might have been due to the recruitment only of patients with advanced stages of AD pathologies.65 This concept encourages the use of CSF biomarkers in prevention clinical trials to test candidate DMT in prodromal and preclinical subjects who will progress to full-blown AD. There are two strategies for using CSF biomarkers in a prevention trial. The first strategy is to use CSF biomarkers as a quantitative measure of pharmacodynamic effects in subjects with genetic risk factors. For example, the phase-2 Alzheimer's Prevention Initiative-Autosomal Dominant Alzheimer's Disease trial [API-ADAD trial (NCT01998841)] for crenezumab (Aβ immunotherapy) and the phase-2/3 API APOE4 trial (NCT02565511) for CAD106 (Aβ immunotherapy) and CNP520 (BACE inhibitor) included PSEN1 E280A carriers and subjects with a homozygous APOE4 genotype, respectively,6667 and incorporated CSF biomarkers such as CSF t-tau or p-tau in the outcome measures.66 In the Dominantly Inherited Alzheimer Network (DIAN) study, the Aβ and tau pathology was expected to start at least 15–20 years before the clinical symptoms.7 Based on the findings of the DIAN study, a phase-2/3 trial of the DIAN Trials Unit (NCT 01760005) for gantenerumab and solanezumab included patients with autosomal dominant APP, PSEN1, and PSEN2 mutations, and Aβ42, t-tau, and p-tau levels in the CSF were measured and correlated with imaging biomarkers as an outcome.68 The second strategy in a prevention trial is to use CSF biomarkers to screen candidates who are likely to progress. For example, the phase-3 Anti-Amyloid treatment in Asymptomatic Alzheimers prevention trial (A4 trial) for solanezumab recruited subjects with evidence of an AP burden in their brain identified through either amyloid imaging scanning or CSF Aβ42 level, and the CSF levels of Aβ and tau were measured as a secondary outcome (NCT02008357). Identifying asymptomatic cognitively normal subjects with AD pathologies is a critical issue for prevention trials, and CSF biomarkers are promising candidates for achieving this. However, sufficient evidence is required that cognitively normal subjects with AD pathologies progress rapidly to MCI and AD compared with subjects without AD pathology. The Alzheimer's Disease Neuroimaging Initiative 3 (ADNI-3) of North America, a project designed to develop CSF biomarkers that support these hypotheses following the results of the previous ADNI-1, ADNI-GO, and ADNI-2 studies, is currently being prepared with the aim of obtaining such evidence.
Prevention trials of other NDs, including PD, have not been proposed because no valid biomarkers associated with disease progression have been identified yet. Therefore, the development of valid biomarkers, particularly CSF biomarkers, will facilitate prevention trials of candidate drugs that target multiple therapeutic targets of NDs in cooperation with genetic and imaging biomarkers.
A surrogate endpoint is defined as a biomarker that is intended to substitute for a clinical endpoint.11 In particular, NDs are chronic diseases with a slow progression of irreversible neuronal damage that is followed by clinical manifestations. Surrogate markers therefore have great value in clinical trials, and CSF biomarkers can be considered as potential surrogate endpoints in an anti-AD trial. However, it should be noted that there is little evidence of CSF biomarkers substituting for clinical endpoints, such as the ADAS-cog, or that the magnitude of changes in CSF biomarkers is correlated with the degree of clinical improvement.69 For example, a phase-3 clinical trial of 18 months of bapineuzumab treatment showed a decrease in the AP burden and CSF p-tau levels, but no clinical benefit.70 Such a mismatch between biomarker changes and clinical efficacy may be attributable to several factors, including the possible time delay between the change in a biomarker to its effect on the clinical course, and the dosing regimen in a long-term trial being insufficient to ensure clinical improvement. Therefore, data supporting clear correlations of CSF biomarkers with clinical outcomes from longer-term observations are required to clarify whether CSF biomarkers can act as surrogate endpoints. Furthermore, the quantitative relationship between the changes in CSF biomarkers and clinical outcome measures needs to be further defined. To this end, several issues related to the measurement of CSF biomarkers are currently being focused on.
The use of CSF biomarkers for the early diagnosis of AD was first proposed more than 2 decades ago, and numerous single-center and multicenter clinical studies have supported the diagnostic potential of CSF biomarkers in AD. The diagnostic criteria for AD have been revised following determinations of successful diagnostic values, although only for research purposes, but including clinical trials. The AD-driven experiences of incorporating CSF biomarkers into clinical trial designs and clinical research will be further extended to other CSF biomarkers and NDs. Global efforts and nationwide clinical research studies have together contributed to the identification of useful AD CSF biomarkers.
However, several issues and limitations remain to be fully resolved. Particularly in clinical trials, appropriate decision-making (e.g., go vs. no-go decisions) is essential for successful development, and so data on the definite roles of CSF biomarkers in clinical trials still needs to be acquired. First, the bias in measuring CSF biomarker levels needs to be clearly defined. The measurement of CSF biomarkers in a central laboratory involving experienced specialists and based on reliable data will be appropriate for the application of CSF biomarkers and the interpretation of data from not only clinical trials but also multicenter studies. In addition, a clinical trial that includes CSF biomarkers should consider a "gray zone" for the cutoff value of CSF biomarkers (Fig. 3). Because there are insufficient data from clinical settings for CSF biomarker-based diagnosis, the inevitable bias present when measuring CSF biomarkers will result in the need for upper and lower cutoff values rather than a single cutoff point. Second, the limitation that changes in CSF biomarkers are at best only weakly correlated with quantified clinical measures should be considered. Because NDs progress slowly, clinical trials covering a period of up to 1 year would not be able to clearly demonstrate correlations of the pathological changes in the drug target as quantified by CSF biomarker levels with clinical outcome measures. Instead, longer-term clinical trials involving multiple doses at the early stages of the disease in pathologically homogeneous patients are necessary to obtain further insight into the relationship. Third, the relationships between CSF biomarkers and other biomarkers (e.g., genetic and imaging) should be defined. The application of multiple biomarkers in clinical trials may improve the power of stratification, interpretability of the data, cost-effectiveness, and cooperative effects. For example, the combined application of CSF biomarkers and the APOE genotype in AD will improve patient stratification and data interpretability. Finally, very few CSF biomarkers have been developed for NDs, except for AD. In particular, the mixed pathology of tauopathy and synucleinopathy in several NDs (e.g., PD dementia) may interfere with the validity of using CSF biomarkers in non-AD NDs. Clinical studies involving autopsy-confirmed patients would be a good strategy for speeding up the development of valid CSF biomarkers. This combined with developing genetic markers will require long-term nationwide and global efforts. The worldwide ADNI and PPMI studies are good examples of the efforts being made for AD and PD, respectively. In addition, the mechanisms of neurodegeneration in NDs should be further investigated using appropriate in vitro and animal models.
In conclusion, CSF biomarkers are beginning to be incorporated in clinical trials of anti-AD DMT. The current data support the potential for the use of CSF biomarkers in clinical trial design and data interpretation. However, the currently available data were obtained in a very limited field of NDs; that is, AD. In addition, several analytical and clinical issues need to be clarified. It is crucial to discover, develop, and use valid CSF biomarkers in clinical trials because valid disease CSF biomarkers have major advantages over clinical measures for the prediction of disease development and/or progression. In addition, collaborative efforts among industry, academia, and regulatory agencies will be important to facilitate the application of CSF biomarkers to the development of DMT.
Figures and Tables
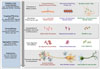 | Fig. 1Illustration of the pathogenesis of neurodegeneration induced by proteinopathy, and the development of drugs targeting this pathogenesis. Amyloid beta (Aβ)-, hyperphosphorylated-tau-, or alpha-synuclein (α-syn)-mediated neurotoxicity is caused by the overproduction of toxic species of the protein from splicing of precursor protein (i.e., amyloid precursor protein) or protein modification (e.g., tau hyperphosphorylation), and/or a decreased clearance of detrimental proteins followed by the production of toxic oligomers and inflammatory microglial activation. Examples of developing drugs targeting the proteinopathy-mediated neurotoxicity are shown. Drugs that are currently being developed include an inhibitor of protein aggregation and antioxidants. |
 | Fig. 2Clinical trials of drugs in Alzheimer's disease (AD) that used cerebrospinal fluid (CSF) biomarkers and are ongoing or were completed within the past 5 years. Each of the developed drugs is arranged according to the estimated study completion date in descending order in each phase. The superscripted letters indicate the specific clinical trials as follows: among solanezumab trials (superscripts d, g, i, and j), (d) and (g) are ongoing prevention trials being performed by the Dominantly Inherited Alzheimer Network (DIAN) Trials Unit and Anti-Amyloid treatment in Asymptomatic AD study (A4 study), respectively, and (i) is another solanezumab trial (EXPEDITION 3) with mild AD that is currently underway after the completion of two double-blind trials (EXPEDITION 1 and 2; superscript j) involving patients with mild-to-moderate AD. One of gantenerumab trials (superscript e) belongs to the DIAN Trials Unit and involves subjects in the preclinical phase of AD, while another (superscript h) is a separate trial of mild AD. Two ongoing trials of IVIG (intravenous immunoglobulin; superscripts a and f) are independent studies supported by different sponsors. Therapeutic targets are presented using different symbols: stars (★) indicate Aβ-producing enzyme, diamonds (♦) indicate Aβ immunotherapy, triangles (►) indicate tau modification or aggregation, cruciform symbols (✚) indicate Aβ aggregation, and closed circles (●) indicate repositioned drug. NCT numbers in the references list indicate the identifiers at www.clinicaltrials.gov. Bars with two colors indicate trials that used CSF biomarkers for two purposes. CD: cluster of differentiation, MCI: mild cognitive impairment, NS: not specified, p-tau: phosphorylated tau, sAPP: soluble amyloid precursor protein, TNF: tumor necrosis factor, t-tau: total tau. |
 | Fig. 3Consideration of a "gray zone" for cutoff values of CSF biomarkers for sample enrichment in clinical trials. The application of a gray zone in which the biomarker values are considered to be inconclusive could be a more realistic approach than the application of single cutoff point in the design of clinical trials. The upper and lower values delimiting the gray zone indicate the 95% confidence interval (95% CI) values calculated using the ADNI-1 cohort (n=116 for controls and n=100 for AD) with cutoff values of 183.5 pg/mL for amyloid beta1–42 (Aβ42) and 86.5 pg/mL for t-tau that were measured using the xMAP-Luminex multiplex platform. The gray zones contain 19% and 19.9% of the biomarker values for CSF Aβ42 and ttau, respectively. Data in the graphs were presented by Coart et al.83 at AAIC 2015 in Washington DC (USA). AD: Alzheimer's disease, CSF: cerebrospinal fluid. |
Table 1
Clinical trials for non-AD NDs that used CSF biomarkers: ongoing or completed within 5 years

| Phase | Drug | Mechanism | CSF biomarker use | Reference* | |
|---|---|---|---|---|---|
| Purpose | Molecules | ||||
| Parkinson disease | |||||
| I | BIIB054 | α-synuclein immunotherapy | Target engagement | α-syn | NCT02459886 |
| I | Nilotinib | Tyrosine kinase inhibitor | Target engagement | α-syn | NCT02281474 |
| I | PD01A | α-synuclein immunotherapy | Target engagement | α-syn | NCT01568099 |
| NCT02216188 | |||||
| I | Inosine | Antioxidant | Target engagement | Urate | Parkinson Study Group SURE-PD Investigators et al.79 |
| II/III | Deferiprone | Iron chelator | Target engagement | Ferritin, HVA/DA, DOPAC/DA | Devos et al.80 |
| I/II | sNN0031 | Platelet derived growth factor | Target engagement | Bilirubin, albumin, PDGF-BB | Paul et al.81 |
| Amyotrophic lateral sclerosis | |||||
| II | Memantine | NMDA receptor antagonist | Target engagement | Tau, pNFH, C3 | NCT02118727 |
| II | Tocilizumab | Immunosuppression | Target engagement | sIL-6 receptor | NCT02469896 |
| II | Basiliximab, methylprednisolone Immunosuppression prednisone tacrolimus mycophenolate mofetil | Immunosuppression | Target engagement | Cytokine | NCT01884571 |
| I | Autologous stem cells | Induce cell replacement | Target engagement | Not molecules† | NCT01609283 |
| II | sNN0029 | Vascular endothelial growth factor | Target engagement | VEGF | NCT01384162 |
| Progressive supranuclear palsy | |||||
| I | BMS-986168 | Tau protein modulator | Target engagement | Extracellular tau | NCT02460094 |
| II/III | Davunetide | Tau protein modulator | Target engagement | Aβ42, t-tau, p-tau, NFH | Boxer et al.82 |
| Progressive supranuclear palsy, frontotemporal dementia, corticobasal degeneration syndrome, progressive nonfluent aphasia | |||||
| II | Davunetide | Tau protein modulator | Target engagement | Aβ42, t-tau, p-tau | NCT01056965 |
*Some trials that have not been published yet, are cited ClinicalTrials.gov numbers, †They include changes in total nucleated cell count and protein level, and presence of cancer cells in CSF.
Aβ: amyloid-β, CSF: cerebrospinal fluid, DA: dopamine, DOPAC: dihydroxyphenylacetic acid, PDGF-BB: platelet-derived growth factor BB, pNFH: Phosphorylated neurofilament heavy protein, p-tau: phosphorylated tau, sIL-6: soluble interleukin 6, t-tau: total tau, VEGF: vascular endothelial growth factor, α-syn: α-synuclein.
Acknowledgements
This work was supported by the Medical Research Center (grant no. 2014009392) and the Original Technology Research Program for Brain Science (grant no. 2014M3C7A1064752) through the National Research Foundation of Korea (NRF) funded by Ministry of Science, ICT and Future Planning, and financially supported by an Inha University Research Grant.
References
1. Wimo A, Jönsson L, Bond J, Prince M, Winblad B. Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013; 9:1–11.e3.

2. Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012; 2:DOI: 10.1101/cshperspect.a006239.

4. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDSADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34:939–944.

5. Blacker D, Albert MS, Bassett SS, Go RC, Harrell LE, Folstein MF. Reliability and validity of NINCDS-ADRDA criteria for Alzheimer's disease. The National Institute of Mental Health Genetics Initiative. Arch Neurol. 1994; 51:1198–1204.

6. Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012; 69:98–106.

7. Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012; 367:795–804.

8. Davies L, Wolska B, Hilbich C, Multhaup G, Martins R, Simms G, et al. A4 amyloid protein deposition and the diagnosis of Alzheimer's disease: prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology. 1988; 38:1688–1693.

9. Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson's disease is not dopamine-mediated. Trends Neurosci. 2003; 26:215–221.

10. de la Fuente-Fernández R. Role of DaTSCAN and clinical diagnosis in Parkinson disease. Neurology. 2012; 78:696–701.

11. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001; 69:89–95.
12. Peskind ER, Riekse R, Quinn JF, Kaye J, Clark CM, Farlow MR, et al. Safety and acceptability of the research lumbar puncture. Alzheimer Dis Assoc Disord. 2005; 19:220–225.

13. Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007; 6:295–303.

14. Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004; 10 Suppl. S10–S17.

15. Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat Cell Biol. 2004; 6:1054–1061.

16. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002; 297:353–356.

17. Vigo-Pelfrey C, Seubert P, Barbour R, Blomquist C, Lee M, Lee D, et al. Elevation of microtubule-associated protein tau in the cerebrospinal fluid of patients with Alzheimer's disease. Neurology. 1995; 45:788–793.

18. Klein WL, Stine WB Jr, Teplow DB. Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol Aging. 2004; 25:569–580.

19. Pyykkö OT, Lumela M, Rummukainen J, Nerg O, Seppälä TT, Herukka SK, et al. Cerebrospinal fluid biomarker and brain biopsy findings in idiopathic normal pressure hydrocephalus. PLoS One. 2014; 9:e91974.

20. Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003; 60:652–656.

21. Hampel H, Bürger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement. 2008; 4:38–48.

22. Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003; 2:605–613.

23. Tapiola T, Overmyer M, Lehtovirta M, Helisalmi S, Ramberg J, Alafuzoff I, et al. The level of cerebrospinal fluid tau correlates with neurofibrillary tangles in Alzheimer's disease. Neuroreport. 1997; 8:3961–3963.

24. Snider BJ, Fagan AM, Roe C, Shah AR, Grant EA, Xiong C, et al. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol. 2009; 66:638–645.

25. Kester MI, van der Vlies AE, Blankenstein MA, Pijnenburg YA, van Elk EJ, Scheltens P, et al. CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology. 2009; 73:1353–1358.

26. Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord. 2007; 24:118–124.

27. Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009; 66:382–389.
28. Kang JH, Korecka M, Toledo JB, Trojanowski JQ, Shaw LM. Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-β(1-42) and τ proteins as Alzheimer disease biomarkers. Clin Chem. 2013; 59:903–916.

29. Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009; 65:403–413.

30. Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement. 2012; 8:65–73.

31. Fagan AM, Perrin RJ. Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer's disease. Biomark Med. 2012; 6:455–476.

32. Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015; 78:787–800.

33. Ishiki A, Okamura N, Furukawa K, Furumoto S, Harada R, Tomita N, et al. Longitudinal assessment of tau pathology in patients with Alzheimer's disease using [18F]THK-5117 positron emission tomography. PLoS One. 2015; 10:e0140311.

34. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997; 388:839–840.
35. Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, et al. Alpha-synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002; 322:1089–1102.

36. Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011; 10:230–240.

37. Mollenhauer B, Trautmann E, Taylor P, Manninger P, Sixel-Döring F, Ebentheuer J, et al. Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett. 2013; 532:44–48.

38. Parnetti L, Chiasserini D, Persichetti E, Eusebi P, Varghese S, Qureshi MM, et al. Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson's disease. Mov Disord. 2014; 29:1019–1027.

39. Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS, et al. Association of cerebrospinal fluid β-amyloid 1-42, T-tau, Ptau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. 2013; 70:1277–1287.

40. Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011; 69:570–580.

41. Parkinson Progression. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011; 95:629–635.
42. Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010; 133(Pt 3):713–726.

43. Kim D, Paik JH, Shin DW, Kim HS, Park CS, Kang JH. What is the clinical significance of cerebrospinal fluid biomarkers in Parkinson's disease? Is the significance diagnostic or prognostic? Exp Neurobiol. 2014; 23:352–364.

44. Levy G, Tang MX, Louis ED, Côté LJ, Alfaro B, Mejia H, et al. The association of incident dementia with mortality in PD. Neurology. 2002; 59:1708–1713.

45. Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008; 70:1017–1022.

46. Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, Chen-Plotkin A, et al. CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010; 75:1055–1061.

47. Alves G, Lange J, Blennow K, Zetterberg H, Andreasson U, Førland MG, et al. CSF Aβ42 predicts early-onset dementia in Parkinson disease. Neurology. 2014; 82:1784–1790.

48. Mattsson N, Rajendran L, Zetterberg H, Gustavsson M, Andreasson U, Olsson M, et al. BACE1 inhibition induces a specific cerebrospinal fluid β-amyloid pattern that identifies drug effects in the central nervous system. PLoS One. 2012; 7:e31084.

49. Forman M, Palcza J, Tseng J, Leempoels J, Ramael S, Han D, et al. The novel BACE inhibitor MK-8931 dramatically lowers cerebrospinal fluid aβ peptides in healthy subjects following single- and multiple-dose administration. Alzheimers Dement. 2012; 8:4 suppl. P704. DOI: 10.1016/j.jalz.2012.05.1900.
50. Forman M, Kleijn H, Dockendorf M, Palcza J, Tseng J, Canales C, et al. The novel BACE inhibitor MK-8931 dramatically lowers CSF beta-amyloid in patients with mild-to-moderate Alzheimer's disease. Alzheimers Dement. 2013; 9:4 suppl. P139. DOI: 10.1016/j.jalz.2013.04.083.
51. Höglund K, Salter H, Zetterberg H, Andreason U, Olsson T, Alexander R, et al. Monitoring the soluble amyloid precursor protein alpha (SAPPA) and beta (SAPPB) fragments in plasma and CSF from healthy individuals treated with bace inhibitor AZD3293 in a multiple ascending dose study: pharmacokinetic and pharmacodynamic correlate. Alzheimers Dement. 2014; 10:4 suppl. P447. DOI: 10.1016/j.jalz.2014.05.605.
52. Alexander R, Budd S, Russell M, Kugler A, Cebers G, Ye N, et al. AZD3293 A novel BACE1 inhibitor: Safety, tolerability, and effects on plasma and CSF aβ peptides following single- and multiple-dose administration. Neurobiol Aging. 2014; 35:suppl 1. S2.

53. Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N Engl J Med. 2013; 369:341–350.

54. Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, et al. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol. 2008; 65:1031–1038.
55. Siemers ER, Quinn JF, Kaye J, Farlow MR, Porsteinsson A, Tariot P, et al. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 2006; 66:602–604.

56. Siemers E, Skinner M, Dean RA, Gonzales C, Satterwhite J, Farlow M, et al. Safety, tolerability, and changes in amyloid beta concentrations after administration of a gamma-secretase inhibitor in volunteers. Clin Neuropharmacol. 2005; 28:126–132.

57. Coric V, van Dyck CH, Salloway S, Andreasen N, Brody M, Richter RW, et al. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol. 2012; 69:1430–1440.

58. Coric V, Salloway S, van Dyck CH, Dubois B, Andreasen N, Brody M, et al. Targeting prodromal Alzheimer disease with avagacestat: a randomized clinical trial. JAMA Neurol. 2015; 72:1324–1333.

59. Dockens R, Wang JS, Castaneda L, Sverdlov O, Huang SP, Slemmon R, et al. A placebo-controlled, multiple ascending dose study to evaluate the safety, pharmacokinetics and pharmacodynamics of avagacestat (BMS-708163) in healthy young and elderly subjects. Clin Pharmacokinet. 2012; 51:681–693.

60. Holland D, McEvoy LK, Desikan RS, Dale AM. Enrichment and stratification for predementia Alzheimer disease clinical trials. PLoS One. 2012; 7:e47739.

61. Blennow K, Zetterberg H. Use of CSF biomarkers in Alzheimer's disease clinical trials. J Nutr Health Aging. 2009; 13:358–361.

62. Andreasen N, Simeoni M, Ostlund H, Lisjo PI, Fladby T, Loercher AE, et al. First administration of the Fc-attenuated anti-β amyloid antibody GSK933776 to patients with mild Alzheimer's disease: a randomized, placebo-controlled study. PLoS One. 2015; 10:e0098153.

63. Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014; 370:311–321.

64. Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, et al. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016; 12:110–120.

65. Vellas B, Carrillo MC, Sampaio C, Brashear HR, Siemers E, Hampel H, et al. Designing drug trials for Alzheimer's disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimers Dement. 2013; 9:438–444.

66. Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, et al. Alzheimer's Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011; 26:Suppl 3. 321–329.

67. Ayutyanont N, Langbaum JB, Hendrix SB, Chen K, Fleisher AS, Friesenhahn M, et al. The Alzheimer's prevention initiative composite cognitive test score: sample size estimates for the evaluation of preclinical Alzheimer's disease treatments in presenilin 1 E280A mutation carriers. J Clin Psychiatry. 2014; 75:652–660.
68. Mills SM, Mallmann J, Santacruz AM, Fuqua A, Carril M, Aisen PS, et al. Preclinical trials in autosomal dominant AD: implementation of the DIAN-TU trial. Rev Neurol (Paris). 2013; 169:737–743.

69. Kang JH, Ryoo NY, Shin DW, Trojanowski JQ, Shaw LM. Role of cerebrospinal fluid biomarkers in clinical trials for Alzheimer's disease modifying therapies. Korean J Physiol Pharmacol. 2014; 18:447–456.

70. Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014; 370:322–333.

71. Bernier F, Sato Y, Matijevic M, Desmond H, McGrath S, Burns L, et al. Clinical study of E2609, a novel BACE1 inhibitor, demonstrates target engagement and inhibition of BACE1 activity in CSF. Alzheimers Dement. 2013; 9:4 suppl. P886. DOI: 10.1016/j.jalz.2013.08.244.
72. Bell J, O'Neill B, Brodney M, Hajos-Korcsok E, Lu Y, Riddell D, et al. A novel BACE inhibitor (PF-05297909): a two-part adaptive design to evaluate safety, pharmacokinetics and pharmacodynamics for modifying beta-amyloid in a first-in-human study. Alzheimers Dement. 2013; 9:4 suppl. P287. DOI: 10.1016/j.jalz.2013.05.578.
73. Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015; 85:1383–1391.

74. Ross J, Sharma S, Winston J, Nunez M, Bottini G, Franceschi M, et al. CHF5074 reduces biomarkers of neuroinflammation in patients with mild cognitive impairment: a 12-week, double-blind, placebocontrolled study. Curr Alzheimer Res. 2013; 10:742–753.

75. Landen J, Cohen S, Billing C, Cronenberger C, Styren S, Burstein A, et al. Safety, efficacy, pharmacokinetics and pharmacodynamics of multiple doses of ponezumab in subjects with mild-to-moderate Alzheimer's disease. Alzheimers Dement. 2012; 8:4 suppl. P708. DOI: 10.1016/j.jalz.2012.05.1913.
76. Landen J, Andreasen N, Cronenberger C, Schwartz P, Börjesson-Hanson A, Östlund H, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of monthly and quarterly doses of ponezumab (PF-04360365) in subjects with mild-to-moderate Alzheimer's disease. Alzheimers Dement. 2012; 8:4 suppl. P708. DOI: 10.1016/j.jalz.2012.05.1914.
77. Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012; 69:29–38.

78. Salloway S, Sperling R, Keren R, Porsteinsson AP, van Dyck CH, Tariot PN, et al. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011; 77:1253–1262.

79. Parkinson Study, Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014; 71:141–150.
80. Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, et al. Targeting chelatable iron as a therapeutic modality in Parkinson's disease. Antioxid Redox Signal. 2014; 21:195–210.

81. Paul G, Zachrisson O, Varrone A, Almqvist P, Jerling M, Lind G, et al. Safety and tolerability of intracerebroventricular PDGF-BB in Parkinson's disease patients. J Clin Invest. 2015; 125:1339–1346.

82. Boxer AL, Lang AE, Grossman M, Knopman DS, Miller BL, Schneider LS, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 2014; 13:676–685.

83. Coart E, Barrado LG, Vanderstichele H, Burzykowski T. The confidence level of established cut-off values for CSF Alzheimer's diseasespecific biomarkers. Alzheimers Dement. 2015; 11:7 suppl. P298. DOI: 10.1016/j.jalz.2015.07.410.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download