Abstract
Background and Purpose
We report a novel finding of caloric conversion from normal responses into unilateral paresis during the acute phase of vestibular neuritis (VN).
Methods
We recruited 893 patients with a diagnosis of VN at Dizziness Clinic of Seoul National University Bundang Hospital from 2003 to 2014 after excluding 28 patients with isolated inferior divisional VN (n=14) and those without follow-up tests despite normal caloric responses initially (n=14). We retrospectively analyzed the neurotological findings in four (0.5%) of the patients who showed a conversion from initially normal caloric responses into unilateral paresis during the acute phase.
Results
In those four patients, the initial caloric tests were performed within 2 days of symptom onset, and conversion into unilateral caloric paresis was documented 1–4 days later. The clinical and laboratory findings during the initial evaluation were consistent with VN in all four patients except for normal findings in bedside head impulse tests in one of them.
Vestibular neuritis (VN) refers to a syndrome of acute unilateral vestibular deafferentation due to inflammation of the peripheral vestibular organ.1 Patients with VN invariably suffer from spontaneous vertigo, nausea/vomiting, and unsteadiness.2 Along with spontaneous horizontal-torsional nystagmus beating toward the side of the healthy ear, identifying unilateral peripheral vestibular hypofunction is the key step for the diagnosis of VN.3 In this regard, unilateral caloric paresis has been the diagnostic hallmark of VN even though unified diagnostic criteria have not been available for this disorder, which is the most-common cause of acute prolonged spontaneous vertigo.1
The increasing awareness of isolated spontaneous vertigo being caused by vascular lesions necessitates a clearer differential diagnosis in this vertigo syndrome, which requires the objective documentation of the findings indicative of peripheral or central vestibulopathy. Thus, the findings of bithermal caloric tests are important in the differential diagnosis since the presence of normal caloric responses can reliably exclude the possibility of peripheral vestibular dysfunction and indicate a central lesion as a cause of acute spontaneous vertigo.4
We report four patients whose caloric responses were initially normal, but became unilaterally paretic several days later during the acute phase of VN. Recognition of these unusual manifestations of caloric responses in VN would aid the differential diagnosis of acute spontaneous vertigo.
We retrospectively reviewed our prospective database that includes all consecutive patients with a diagnosis of unilateral VN at the Dizziness Clinic of Seoul National University Bundang Hospital from May 2003 to November 2014. The diagnosis of unilateral VN was based on the findings of a bedside examination and laboratory evaluation that include 1) acute spontaneous vertigo lasting more than 12 hours with unidirectional spontaneous nystagmus (SN, mostly horizontal-torsional), 2) no auditory symptoms including tinnitus, ear fullness, or hearing impairment, or hearing loss documented with pure-tone audiometry (PTA), 3) no additional neurological signs or symptoms suggestive of a central lesion, and 4) a canal paresis of 25% or more on bithermal caloric tests or unilaterally positive head-impulse tests (HITs) for any of three semicircular canals (SCCs). The diagnosis of VN was made when the patients fulfilled all four of these criteria.
Bithermal caloric tests were performed in all 921 patients with a diagnosis of VN during the study inclusion period. Among them, 32 patients showed normal caloric responses initially, and finally 4 patients with a later conversion into unilateral caloric paresis were included in this study after excluding the patients with isolated inferior divisional VN (n=14), and those who did not receive follow-up tests despite exhibiting normal caloric responses initially (n=14). The diagnosis of isolated inferior divisional VN was based on 1) symptoms of acute unilateral vestibulopathy, 2) spontaneous torsionaldownbeat nystagmus, 3) abnormal HIT findings for the posterior semicircular canals (PCs) but normal HIT findings for the anterior (ACs) and horizontal semicircular canals (HCs), 4) abnormal cervical vestibular-evoked myogenic potentials (cVEMPs), 5) normal findings of caloric tests, and 6) exclusion of central lesions using brain MRI and neurological examinations.5
The bedside neurotological examination included evaluations of SN with and without visual fixation, gaze-evoked nystagmus (GEN), head-shaking (HSN), vibration-induced (VIN), and positional nystagmus without fixation, ocular misalignment including skew deviation, head tilt, and eye movements including smooth pursuit, saccades and the vestibulo-ocular reflex (VOR) as measured with HITs. For HITs, the patients were instructed to fixate on the examiner's nose. Meanwhile, the patient's head was rotated rapidly by approximately 20° in the planes of the SCCs in a passive, unpredictable manner so as to preclude any prediction. The results were considered to be positive only if corrective saccades were elicited repetitively.6
The eye movements were also assessed quantitatively using video-oculography (SMI, Teltow, Germany). SN was recorded with and without visual fixation while sitting. SN was consider to be abnormal only when it was present even during visual fixation or when the slow-phase velocity (SPV) of the SN exceeded the values observed in normal controls (≥1.1°/s) without fixation. HSN and VIN were considered to be present only when the maximal SPV exceeded the values (mean+2 SDs) observed in normal controls after subtracting the SPV of SN, and when it lasted more than 5 s.7
We also measured the HITs using a magnetic search coil (MSC) technique in two patients (patients 1 and 2). A scleral annulus ring (CHRONOS VISION, Berlin, Germany) was placed on the subject's left eye after anesthetizing the conjunctiva with 0.5% proparacaine hydrochloride (Alcon, Seoul, Korea). A second coil was fixed at the center of the forehead. The eye and head positions were calibrated with a gimbal that could be rotated independently around the three orthogonal axes. In addition to this in-vitro calibration using the gimbal system, we also used horizontal (-10° to +10°) and vertical (-10° to +10°) fixation spots for in-vivo eye calibration. Patients were instructed to fixate on a red target placed 1.2 m in front of them. The head impulses comprised passive, unpredictable, low-amplitude, and high-acceleration head rotations in the planes of both HCs, the left AC and right PC, and the right AC and left PC while the patients sat upright. The eye and head position signals were digitized at 200 Hz by an analog-to-digital converter (EZAD, Seoul, Korea) and displayed on a computer screen to allow eye motion to be monitored during the testing. Digitized data were analyzed with MATLAB software (version R2011b, The MathWorks, Natick, MA, USA).8 At least seven impulses were delivered in each direction. The gain of the VOR was calculated for each trial as the ratio of the mean velocity of the eye divided by the mean velocity of the head during a 40-ms window centered at the time of peak head acceleration.91011
Reference data were obtained in 19 healthy subjects [14 women; age=44±13 years (mean±SD), age range=24–66 years] with no history of vestibular or neurological disorders. We defined the HIT findings as being abnormal when the mean VOR gain was less than the mean-2 SDs of the control data (<0.70 for the HC, <0.70 for the AC, and <0.74 for the PC).
After examining the tympanic membranes and determining the presence of SN, bithermal caloric tests were performed by irrigating the ears for 25 s alternately with 150–250 mL of cold (30℃) and hot (44℃) water. The induced nystagmus was recorded with video-oculography either monocularly (ICS Medical, Schaumburg, IL, USA) or binocularly (Neurokinetics, Pittsburgh, PA, USA) until it had decayed to the null. The patients were supine while wearing video-oculography goggles in a dark room throughout the measurements. Asymmetry of vestibular function was calculated using Jongkees' formula. Canal paresis was defined as a response difference of 25% or more between the ears.12
A 72-year-old woman with hypertension presented with vertigo and imbalance that had been present for 1 day. She had undergone a lumpectomy due to breast cancer 4 years previously. She denied any diplopia, tinnitus, aural fullness, or hearing impairments. Examination showed spontaneous right-beating horizontal-torsional nystagmus with an upward component under Frenzel goggles. Bedside HITs were normal in both horizontal directions. She did not show GEN, head tilt, or skew deviation. Remainder of the neurological examination was unrevealing.
Bithermal caloric tests produced normal findings, without asymmetry (Fig. 1A). Video-oculography performed 2 days after symptom onset also documented the SN that had been observed at the bedside (Fig. 1B). Vibratory stimuli to either mastoid augmented the SN, but the SN was not significantly altered by horizontal HSN.7 oVEMPs showed a decrease in the N1-P1 amplitude during left-ear stimulation with an asymmetry ratio of 0.36 (normal range: ≤0.22) (Fig. 1C). She also exhibited counterclockwise tilt of the SVV while left monocular viewing (-8.8°, normal range: -3.1° to 3.0°; negative value indicates a counterclockwise tilt) and binocular viewing (-3.4°, normal range: -3.0° to 2.2°).12 However, cVEMPs and PTA were normal. In view of the normal bedside HITs and caloric tests in the presence of acute spontaneous vertigo and nystagmus, brain MRI including diffusion-weighted sequences was performed, but this did not reveal lesions responsible for her symptoms and signs.
The vertigo and SN did not change significantly during the following 4 days, but a follow-up evaluation showed a positive bedside HIT to the left, HSN to the right, abnormal extorsion of the left eye (Fig. 1D) (21.6°, normal range: 0–12.6°; positive value indicates extorsion), counterclockwise (from the patient's perspective) SVV tilt, and complete left caloric paresis (Fig. 1A). Recording of HITs using the MSC technique documented decreased gains for the left HC (gain=0.13) and AC (gain=0.33), but normal gains for other SCCs including the left PC (gain=0.99) (Fig. 1E).8 Her vertigo and imbalance had resolved over the following 5 days, and no symptoms recurred during a 2-month follow-up.
The bedside and laboratory neurotological findings of our patients are summarized in Fig. 2. All four patients presented with isolated acute vestibular syndrome characterized by spontaneous vertigo and unsteadiness for 1 day. Two patients had a previous medical history of hypertension. None of the patients was taking any medication that could influence the results of the vestibular function tests. During the initial evaluation performed within 2 days of symptom onset, all patients showed unidirectional horizontal-torsional SN with (n=3) or without an upbeat component (n=1) that followed Alexander's law (i.e., more intense during the gaze in the direction of nystagmus and smaller in the opposite direction) without any direction-changing. Vibratory stimuli to either mastoid usually increased the contralesional SN (n=3), but horizontal HSN did not change the SN significantly in most patients (n=3). Positional maneuvers did not alter the SN significantly. Bedside HITs were positive in the direction opposite to SN in three patients, but were considered to be normal in the remaining one (patient 1). Three patients (patients 1, 3, and 4) showed tilt of SVV and ocular torsion in the direction opposite to SN, but none of the patients exhibited skew deviation. Smooth pursuit was saccadic only in the direction of SN, and saccades were normal in all patients. oVEMPs were abnormal in one of the three patients who was tested, while the cVEMPs were normal in all four patients. These findings were mostly consistent with VN (Table 1).
However, the patients showed normal caloric responses, and brain MRI including diffusion-weighted sequences did not reveal any abnormalities responsible for the acute spontaneous vestibular syndrome. Given the negative imaging findings, follow-up caloric tests were performed 1–4 days later, which eventually documented unilateral caloric paresis. In one patient (patient 1) with initially normal findings in bedside HITs and caloric tests, recording of HITs using the MSC technique 4 days later documented decreased gains for HC and AC on the lesion side along with a conversion into caloric paresis. In the other patient (patient 2), the recording of HITs using an MSC technique 5 days after symptom onset confirmed that the head impulse VOR gain was decreased only in the HC in the presence of caloric paresis.
To the best of our knowledge, this is the first report of initially normal caloric responses that subsequently converted into unilateral paresis during the acute phase of VN.
The caloric test evaluates the VOR by creating a temperature gradient in the SCCs, which induces movement of the endolymph.14 Despite using nonphysiological low-frequency stimuli (<0.003 Hz), the caloric test enables the function of each labyrinth to be assessed separately, and also to differentiate peripheral from central vertigo. The findings of a caloric test are invariably abnormal during the acute stage of VN, and the abnormality is usually maintained for more than 1 year.12 Indeed, unilateral caloric paresis has been considered to be the diagnostic hallmark of VN.12
The caloric responses in our patients with a final diagnosis of VN were initially symmetrical even in the presence of SN. This indicates dissociation between the static and dynamic vestibular imbalances during the acute phase of VN. Moreover, three of them (patients 2, 3, and 4) showed abnormal HITs but normal caloric responses. This further implies that the dynamic vestibular responses may vary according to the characteristics of the stimuli in peripheral1215 as well as central vestibular disorders.
The primary vestibular afferents are categorized according to their spontaneous firing patterns.14 The regular afferents mainly encode the low-frequency movements of the endolymph (e.g., caloric responses), and innervate either the type II cells or a mixture of type I and type II cells. In contrast, the irregular afferents are known to encode higher frequency stimuli (e.g., head impulses), and innervate either the type I cells or a mixture of type I and type II cells. However, histopathological studies of VN have not shown any predilection for a specific type of hair cells.1617 In addition, VN patients mostly show impaired responses on both caloric and HITs.18 Thus, the pattern of dissociation in vestibular dysfunction observed in our patients indicates a possibility of relative sparing of the low-frequency vestibular afferents (<0.003 Hz) during the early evolution of the inflammation involving the vestibular nerve in some patients.
Indeed, the vestibular asymmetry may build up in a gradual manner in VN. The recovery of vestibular imbalances also occurs on different timescales according to the stimuli applied in VN; that is, the caloric asymmetry outlasts the abnormalities of the HITs, HSN, and VIN, which employ stimulus frequencies higher than that used in the caloric tests.1218 Likewise, the current study showed that vestibular abnormalities may emerge on different timescales during the acute phase of VN. Indeed, a previous study found that dizziness occurred within 1 week prior to a VN attack in 24% of patients,19 which is also indicative of a gradual or stepwise buildup of the vestibular asymmetry in VN. However, normal caloric responses should be rare in VN, if they occur at all, since only 18 (1.9%) of the present 907 patients showed normal results when 14 patients with an initial diagnosis of isolated inferior VN were excluded.
The subsequent conversion into unilateral caloric paresis has been described previously in patients with a progression from isolated inferior VN into labyrinthitis involving the entire vestibular and auditory compartments.5 Accordingly, the initially normal caloric responses in those patients may be ascribed to sparing of the HC that belongs to the superior division. However, we excluded patients with an initial diagnosis of isolated inferior VN in this study, and the included only patients who showed findings consistent with an involvement of the superior vestibular division from the beginning. Thus, the initially normal caloric responses cannot be explained by a selective sparing of the HC function in our patients.
Various factors could potentially influence the magnitude of caloric responses, such as visual fixation, the alertness or psychological status of the patient,20 blood flow to the skin,21 heat conductivity of the temporal bone,21 and even test repetition. 22 Thus, caloric responses should be interpreted with caution due to both interindividual and intraindividual variability. In our patients, the caloric tests were repeated using the same methods and equipment over a short period of time by the same technician. Furthermore, repeated caloric testing tends to decrease the caloric asymmetry,22 which was not the case in our patients. In addition, the caloric responses might be underestimated when the SN is too strong to be reversed by caloric stimulation. To avoid this, we thoroughly reviewed the raw SPV data in each patient. In addition, two patients showed a relatively small SN (<3.0°/s) that would not have influenced the caloric responses. However, intraindividual variability of the caloric responses remains a possibility in our patients.
Differentiating between central and peripheral etiologies in acute isolated spontaneous vertigo is important since 10–20% of patients experiencing posterior circulation strokes may present with isolated vertigo (pseudo-VN).23 Since the patterns of SN alone cannot differentiate peripheral from central causes of vertigo, other vestibular findings should be carefully investigated to secure differential diagnosis.24 In this regard, the findings of HITs and caloric tests are important since pseudo-VNs due to lesions mostly involving the vestibulocerebellum almost always show normal HITs and caloric responses.23 The HITs and caloric tests are complementary in detecting peripheral vestibular hypofunction, since HIT findings may be normal in patients with incomplete peripheral vestibular damage. Indeed, the sensitivity of bedside HITs is reportedly 63–72%.25 Given the relatively older ages and comorbid vascular risk factors in our patients, their acute spontaneous vertigo might be attributable to vascular inner ear damage. However, labyrinthine infarctions tend to involve both the vestibular and cochlear end organs, and isolated damage to the vestibular labyrinth would be extremely rare (if it occurs at all) in the presence of vascular inner ear damage.26
In conclusion, the results of caloric tests should be interpreted with caution during the acute phase of spontaneous vertigo since VN patients can present with normal caloric responses during the hyperacute phase. Therefore, follow-up caloric tests should be considered when the initial evaluation is normal, but VN remains the most plausible diagnosis.
Figures and Tables
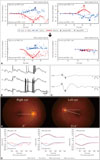 | Fig. 1Neurotological findings in patient 1. A: The initially normal caloric responses changed into complete left paralysis 4 days later. B: Video-oculography shows spontaneous nystagmus (SN) beating rightward, upward, and clockwise (from the patient's perspective). C: Ocular vestibular evoked myogenic potentials were decreased during left-ear stimulation. LH: horizontal position of the left eye, LV: vertical position of the left eye, LT: torsional position of the left eye, PSPV: peak slow-phase velocity. D: Fundus photography shows extorsion of the left eye (normal range: 0–12.6°, positive value indicates extorsion). E: Magnetic search coil recordings of head impulse tests show decreased gains for the left horizontal (HCs) and anterior canals (ACs), but normal gains for other semicircular canals including the left posterior canal (PC). LAC: left AC, LHC: left HC, LPC: left PC, RAC: right AC, RHC: right HC, RPC: right PC. |
 | Fig. 2Evolution of the bedside and laboratory neurotological findings in each patient. *Day 0: the day of symptom onset, †Evaluations using a magnetic search coil (MSC) technique. cVEMP: cervical vestibular-evoked myogenic potential, HIT: head-impulse test, HSN: head-shaking nystagmus, oVEMP: ocular vestibular-evoked myogenic potential, SN: spontaneous nystagmus, SVV: subjective visual vertical, VIN: vibration-induced nystagmus. |
Table 1
Clinical features of the patients with vestibular neuritis who showed caloric conversion

Acknowledgements
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (no. HI10C2020).
Notes
Conflicts of Interest Drs. Lee, Park, Koo, and H.J. Kim have no potential conflicts of interest to disclose. Dr. J.S. Kim serves as an associate editor of Frontiers in Neurootology and on the editorial boards of the Journal of Korean Society of Clinical Neurophysiology, Journal of Clinical Neurology, Frontiers in Neuro-ophthalmology, Journal of Neuro-ophthalmology, Journal of Vestibular Research, Journal of Neurology, and Medicine, and received research support from SK Chemicals, Co. Ltd.
References
3. Baloh RW. Clinical practice. Vestibular neuritis. N Engl J Med. 2003; 348:1027–1032.
4. Cnyrim CD, Newman-Toker D, Karch C, Brandt T, Strupp M. Bedside differentiation of vestibular neuritis from central "vestibular pseudoneuritis". J Neurol Neurosurg Psychiatry. 2008; 79:458–460.

7. Choi KD, Oh SY, Park SH, Kim JH, Koo JW, Kim JS. Head-shaking nystagmus in lateral medullary infarction: patterns and possible mechanisms. Neurology. 2007; 68:1337–1344.

8. Choi KD, Oh SY, Kim HJ, Kim JS. The vestibulo-ocular reflexes during head impulse in Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry. 2007; 78:1161–1162.

9. Chen L, Todd M, Halmagyi GM, Aw S. Head impulse gain and saccade analysis in pontine-cerebellar stroke and vestibular neuritis. Neurology. 2014; 83:1513–1522.

10. Lehnen N, Aw ST, Todd MJ, Halmagyi GM. Head impulse test reveals residual semicircular canal function after vestibular neurectomy. Neurology. 2004; 62:2294–2296.

11. Kim HJ, Park SH, Kim JS, Koo JW, Kim CY, Kim YH, et al. Bilaterally abnormal head impulse tests indicate a large cerebellopontine angle tumor. J Clin Neurol. 2016; 12:65–74.

12. Choi KD, Oh SY, Kim HJ, Koo JW, Cho BM, Kim JS. Recovery of vestibular imbalances after vestibular neuritis. Laryngoscope. 2007; 117:1307–1312.

13. Kim S, Lee HS, Kim JS. Medial vestibulospinal tract lesions impair sacculo-collic reflexes. J Neurol. 2010; 257:825–832.

14. Baloh RW, Kerber KA, Honrubia V. Clinical Neurophysiology of the Vestibular System. 4th ed. New York, NY: Oxford University Press;2011.
15. Maire R, van Melle G. Vestibulo-ocular reflex characteristics in patients with unilateral Ménière's disease. Otol Neurotol. 2008; 29:693–698.

16. Nadol JB Jr. Vestibular neuritis. Otolaryngol Head Neck Surg. 1995; 112:162–172.
17. Schuknecht HF, Kitamura K. Second Louis H. Clerf Lecture. Vestibular neuritis. Ann Otol Rhinol Laryngol Suppl. 1981; 90(1 Pt 2):1–19.
18. Schmid-Priscoveanu A, Böhmer A, Obzina H, Straumann D. Caloric and search-coil head-impulse testing in patients after vestibular neuritis. J Assoc Res Otolaryngol. 2001; 2:72–78.

19. Lee H, Kim BK, Park HJ, Koo JW, Kim JS. Prodromal dizziness in vestibular neuritis: frequency and clinical implication. J Neurol Neurosurg Psychiatry. 2009; 80:355–356.

20. Proctor L, Glackin R. Factors contributing to variability of caloric test scores. Acta Otolaryngol. 1985; 100:161–171.

21. Baertschi AJ, Johnson RN, Hanna GR. A theoretical and experimental determination of vestibular dynamics in caloric stimulation. Biol Cybern. 1975; 20:175–186.

22. Furman JM, Jacob RG. Jongkees' formula re-evaluated: order effects in the response to alternate binaural bithermal caloric stimulation using closed-loop irrigation. Acta Otolaryngol. 1993; 113:3–10.

23. Lee H, Sohn SI, Cho YW, Lee SR, Ahn BH, Park BR, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology. 2006; 67:1178–1183.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download