Abstract
Intracranial arterial calcification (IAC) is an easily identifiable entity on plain head computed tomography scans. Recent studies have found high prevalence rates for IAC worldwide, and this may be associated with ischemic stroke and cognitive decline. Aging, traditional cardiovascular risk factors, and chronic kidney disease have been found to be associated with IAC. The severity of IAC can be assessed using different visual grading scales or various quantitative methods (by measuring volume or intensity). An objective method for assessing IAC using consistent criteria is urgently required to facilitate comparisons between multiple studies involving diverse populations. There is accumulating evidence from clinical studies that IAC could be utilized as an indicator of intracranial atherosclerosis. However, the pathophysiology underlying the potential correlation between IAC and ischemic stroke—through direct arterial stenosis or plaque stability—remains to be determined. More well-designed clinical studies are needed to explore the predictive values of IAC in vascular events and the underlying pathophysiological mechanisms.
Vascular calcification appears to be specific for arteries, which can involve the femoral artery, iliac artery, abdominal aorta, thoracic aorta, coronary artery, carotid artery, and cerebral arteries. Histologically confirmed arterial calcification can occur in localized intimal plaques, as well as in a diffused fashion in the media (Mönckeberg's medial calcification).123 Intimal calcification involves a complex, regulated process of biomineralization resembling osteogenesis,4 and the current review focuses on vascular calcification.
According to the literature, vascular calcification is considered to be an integral part of the active process of atherosclerosis, occurring in up to 90% of atherosclerotic lesions.5 The biological mechanism underlying the development of calcification is complex and is not well understood. Vascular calcification is beginning to be regarded as regulated ossification that includes both osteogenic and chondrogenic differentiation, because ossification has been identified histologically as occurring at different locations of vascular calcification, such as aortic valves, carotid atherosclerotic plaque specimens, and calcified cardiac valve tissue. Many key regulators of bone formation and bone structural proteins are expressed in atherosclerotic plaques, including bone morphogenetic protein-2,6 osteopontin,7 matrix γ-carboxyglutamic acid protein, and osteoprotegerin.8
Coronary artery calcification (CAC) is by far the best-studied type of vascular calcification.9 A CAC score has been used as a noninvasive marker to predict acute coronary disease, and this has prompted researchers to explore the potential predictive values of calcium scores in other arteries.
Intracranial arterial calcification (IAC) was first observed in the early 1960s using ex vivo radiography and microscopic pathology investigations.101112 Although IAC is frequently observed on unenhanced head computed tomography (CT) and is easy to identify, its clinical significance has long been neglected. The establishment of intracranial artery atherosclerosis as a major cause of ischemic stroke worldwide has increased the interest of researchers in IAC, which is an important component in advanced intracranial atherosclerosis. Several clinical studies have found a high prevalence of IAC in Asians and Caucasians.1314
In this review we document clinical aspects of IAC, including its risk factors, distributions in different cerebral arteries, the currently available methods for assessing the severity of IAC, and possible mechanisms underlying the effects of IAC on ischemic stroke (arterial stenosis or plaque stability).
As a proxy of atherosclerosis, IAC shares some traditional cardiovascular risk factors with other atherosclerotic diseases. The first study of IAC did not observe intimal calcification in any patient younger than 20 years, and therefore the condition was considered to be associated with the aging process. Several studies that have utilized various methods to measure the severity of calcification have demonstrated a trend for IAC to increase with age.131415 Being male is another factor that may be associated with IAC,16 as supported by the population-based Rotterdam Study involving 2,495 subjects.17 Table 1 lists other vascular factors that may be associated with IAC, including hypertension, diabetes, hypercholesterolemia, and ischemic stroke. Besides cardiovascular risk factors, the occurrence of calcification is also found to be related to calcium-phosphate imbalance. A high prevalence of IAC was reported in patients with chronic kidney disease,181920 possibly due to the calcium-phosphate imbalance resulting from renal failure.
Calcification in the intracranial internal carotid artery (IICA) segment (cavernous carotid or carotid siphon) is the most commonly reported location in the literature.21222324252627282930 X-ray examinations of postmortem IICA specimens performed in 1965 by Fisher et al.11 detected 75% calcification in these vessel specimens. Recent clinical studies involving community or stroke patients have consistently found the IICA to be the most common site of IAC. The incidence of IICA calcification has been reported to range from 60% to 90% depending on age, ethnicity, and the presence of stroke or other risk factors.
The vertebral artery is the second most common artery affected by calcification.1213 Calcification was identified in one or both intracranial vertebral arteries in 3.4% of 3,648 CT scans,31 and was more frequent in the higher age groups. Another study that involved 490 Chinese hospital patients found a higher incidence of intracranial vertebral artery calcification (20%).13 Compared to an incidence of 3–20% in all patients, Pikija et al.32 reported a much higher incidence of around 70% calcification of bilateral vertebral arteries among stroke patients, in addition to 17.1% calcification in the basilar artery.
These reports indicate that the IICA, vertebral artery, and basilar artery are often affected by calcification. Chen et al.13 reported a similar incidence of calcification (5%) in the middle cerebral artery and basilar artery. In contrast, the anterior and posterior cerebral arteries are seldom affected by calcification.
Vascular calcification can be quantified in vivo or in vitro using diverse methods.33 Besides an in vitro histological test of vessel specimens, an unenhanced CT scan is the most accessible and direct method to evaluate IAC in patients. The advent of multidetector CT technology has greatly increased the accuracy and sensitivity of delineating IAC.34 CT angiography (CTA) is also a common method for investigating the cerebral vasculature. However, Ahn et al.28 from Korea suggested that unenhanced CT would be a better approach for evaluating IAC since it could more readily demonstrate small amounts of calcification. Although magnetic resonance imaging (MRI) is not a good choice for IAC evaluations because of its relatively high cost and technological requirements, recent advances in MRI technology have made it feasible to identify calcification components from plaque components, such as fibrous tissue, lipids, and thrombus. In this review we focus on methods for evaluating IAC utilizing CT and CTA imaging due to their wide availability in general hospitals.
A search of the relevant literature revealed that the current methods used to evaluate the severity of IAC are both diverse and inconsistent (Table 2). Visual grading systems are the most common qualitative methods used to assess IAC13142223 despite the large variations in the obtained results depending on which factors are considered and which grading criteria are applied. The extent and thickness of calcification are the two main factors graded by visual inspection35 (Fig. 1). The sum of the grading values in individual cerebral arteries is used to represent the degree of calcification in the entire intracranial vasculature.3637 However, the subjective nature and inconsistencies between different grading scales hinder comparisons of findings from different studies. Therefore, accurate and quantitative measurements of IAC are required to facilitate the performing of IAC-related clinical trials (Fig. 2).
With the aim of supporting quantitative measurements, de Weert et al.16 developed a semiautomatic custom-made system for evaluating IAC. The volume of calcifications was measured with the freely available ImageJ software using a custom-made polymeasure plug-in. The volume of calcifications was calculated by multiplying the number of pixels above a preset threshold, the pixel size, and the increment. The feasibility of such volume measurement was verified by its strong correlation with qualitative calcium categorizations. However, the close spatial relationship between calcification located in the intracranial arterial wall and the bony structures of the skull base is a major hurdle for semiautomatic measurements of IAC. By using manual tracing to separate the calcium in the vessel wall from that in the skull, Bleeker et al.27 verified the reliability of their measurements with excellent inter-observer agreement (Pearson correlation coefficient=0.99) and a strong correlation with qualitative IAC grading results.
The Agatston score is a well-known and validated quantitative parameter adopted to assess calcium in the coronary artery, which is defined as the area of calcification (determined by a certain threshold value) multiplied by a weighted value assigned to its highest value in Hounsfield units.9 Inspired by the clinical application of the Agatston score in evaluating CAC, Taoka et al.24 used the Agatston score to evaluate carotid siphon calcification, and found that the score was strongly correlated with arteriosclerotic changes both in the carotid siphon and the bifurcation. Considering the challenges in segmenting IAC from the neighboring bony structures, special techniques are required to eliminate the contamination of bone attenuation when obtaining quantitative scores of IAC.24
Several measurement tools are available for evaluating the severity of IAC, including subjective visual grading scales and the relatively objective quantitative measurements. Due to inconsistent criteria used when grading IAC, a quantitative and reproducible measuring tool is urgently needed for facilitating comparisons between different studies.
Arterial stiffening is a phenomenon associated with aging and accelerated by other vascular risk factors, which can be evaluated by the pulse wave velocity (PWV) and may indicate early atherosclerotic changes. In coronary artery disease, the degree of CAC was found to be significantly related to the aortic PWV, which is a measure of central arterial stiffness.38 Several studies have verified the correlation between IAC and arterial stiffness. Among community-based Chinese patients without stroke, asymptomatic intracranial artery atherosclerosis (defined as intracranial arterial stenosis and/or IAC) was demonstrated to be associated with large-artery stiffness, as measured by the carotid-femoral PWV and 24-h ambulatory pulse pressure.39 In a Korean study, Park et al.40 also found that increased brachial-ankle PWV was closely associated with the degree of IAC in patients with acute ischemic stroke. By performing transcranial Doppler ultrasonography in the cerebral vasculature, those authors found that the severity of IAC was correlated with the pulsatility index of intracranial vessels in stroke patients.40 Therefore, IAC may increase both the systemic and intracranial arterial stiffnesses in patients with and without cerebrovascular diseases. Larger studies are needed to further clarify the effect of IAC on cerebral hemodynamics.
Arterial calcification is an indicator of atherosclerosis, which may lead to ischemic stroke due to direct luminal stenosis. Several studies have explored the correlations between IAC and the severity of luminal narrowing. Severe calcification was demonstrated to be correlated with a carotid siphon stenosis of greater than 50%, as determined angiographically.21 Another study used digital subtraction angiography to determine the presence of high-grade stenosis, and verified the correlation between cerebral artery stenosis and IAC.41 Sohn et al.15 found that IAC was associated with a higher degree of intracranial artery stenosis, although they failed to demonstrate any correlation between IAC and an abnormal cerebral blood flow velocity when using transcranial Doppler ultrasonography. However, the retrospective design of these studies and their qualitative assessments of IAC adversely affect the reliability of identifying a correlation between IAC and vascular stenosis. Large prospective studies employing more accurate and quantitative measurement tools are still required to confirm the association between IAC and arterial stenosis.
The contributions of IAC to atherosclerotic plaque stability within the cerebral vasculature are still controversial in advanced atherosclerosis. Due to limited data concerning IAC and plaque stability, we searched relevant studies in the area of CAC. Huang et al.42 used a biomechanical model to test the hypothesis that calcification influences the biomechanical stresses in human atherosclerotic lesions. However, they found that CAC within a rupture or a stable human atherosclerotic plaque did not increase the fibrous cap stress. Another study using multislice CT identified that the prevalence of noncalcified coronary atherosclerotic plaques was higher in patients with acute coronary disease than in those with a stable clinical presentation.43 Similarly, intravascular ultrasound studies found heavily calcified plaques to be more resistant to plaque progression (changes in atheroma volume).44 Therefore, the findings for CAC suggest that heavy calcification may actually help to stabilize atherosclerotic plaques, rather than the other way around. A heavy plaque burden hidden in heavily calcified arteries—rather than plaque vulnerability—may partially account for the association between severe arterial calcification and ischemic events.45 Beside CAC, the histology findings of carotid plaques obtained during carotid endarterectomy procedures also demonstrated that symptomatic plaques are less calcified than asymptomatic plaques, suggesting that carotid plaque calcification is associated with plaque stability.46 However, as for intracranial arteries, a postmortem study involving Chinese patients failed to demonstrate any correlation between IAC and brain infarction in the corresponding vascular territory.47 According to our current knowledge of stroke mechanisms in large-artery atherosclerosis patients, hemodynamic failure secondary to arterial stenosis or artery-to-artery emboli accounts for a high proportion of ischemic stroke events. However, it is still too early to draw any definitive conclusions regarding the relationship between IAC and plaque stability due to the lack of reliable evidence.
As an indicator of atherosclerosis in intracranial arteries, IAC is an easy-to-acquire and promising biomarker reflecting the severity of intracranial vascular disease. The interest of researchers in the clinical significance of IAC has increased due to findings suggesting that moderate-to-extensive CAC is associated with an increased occurrence of ischemic stroke or cerebral small-vessel disease, and that the CAC score may be utilized to evaluate the risk of cerebrovascular diseases.4849
In terms of the clinical significance of IAC, Chen et al.50 were the first to demonstrate in a cross-sectional study that the incidence of IAC was higher in Chinese ischemic stroke patients. However, a subsequent study involving Chinese stroke patients did not find that IAC was an independent predictor of recurrent stroke at 3 years after an index stroke, although this negative result was possibly due to smallness of the sample (n=60).10 Taoka et al.24 found that the degree of calcification in the siphon could be used to indicate angiographic changes of arteriosclerosis but could not predict the possibility of a future cerebral stroke in a Japanese population. In addition to these studies involving Asians, a French research group recruited 302 stroke patients over 1 year and found that IAC detection may constitute a simple marker of a high risk of future major clinical events, including ischemic stroke and death.37 Similarly, Ovesen et al.51 demonstrated that the severity of intracranial atherosclerosis (graded as the number of calcified cerebral arteries) detected during an acute evaluation predicted an increased risk of recurrent stroke in a Danish population (n=652). The recent Rotterdam Study—the largest population-based cohort study in a general community recruiting 2,323 stroke-free elderly subjects—established intracranial atherosclerosis (quantified by the IICA calcification volume on CT scans) to be a major risk factor for stroke in a White population, and suggested that its contribution to the proportion of all strokes may be greater than that of large-artery atherosclerosis in other more proximally located vessel beds.52 The strong association between IICA calcification and stroke demonstrated by the Rotterdam Study revealed that IICA calcification is an important but previously seldom-considered risk factor for subsequent stroke in White patients. The apparent discrepancy between this finding and the previous reports of a low frequency of stroke caused by intracranial atherosclerosis in White patients should reawaken the interests of researchers in intracranial atherosclerosis as a cause of stroke in White individuals.
Ischemic stroke is well known to be a heterogeneous disease attributable to various causes such as large-artery atherosclerosis, cardioembolism, and small-vessel occlusion. However, few studies have explored the associations between IAC and specific stroke subtypes. A subgroup analysis based on the Rotterdam Study aimed to identify how the extent of arterial calcifications differed between nonlacunar and lacunar ischemic strokes.53 Nonlacunar cerebral infarcts were presumed to be caused by thromboembolism from the heart or extracranial arteries, whereas lacunar infarcts were thought to be caused by small-vessel disease. The subgroup analysis involved 820 consecutive patients with transient ischemic attack or ischemic stroke in the anterior circulation, and it demonstrated that the only difference between nonlacunar and lacunar strokes was a higher calcification volume in the aortic arch in nonlacunar strokes, instead of that in intracranial arteries. These findings only partially confirmed the notion of distinct etiologies, suggesting that other plaque components, plaque morphology, and aortic arch calcifications play roles in various ischemic stroke subtypes and so are worthy of further evaluation. Future studies are therefore required to investigate the potential contributions of IAC in different stroke subtypes.
In addition to ischemic stroke, some researchers began to study the relationship between severities of IAC and other coexistent radiologically identified vascular changes. A Korean study involving 445 patients found that higher carotid siphon calcification scores were associated with higher rates of lacunar infarction.54 Similarly, IICA calcification was found to be an independent risk factor for cerebral microbleeds, especially in deep cerebral microbleeds.55 Chung et al.26 found that IICA calcification was associated with whitematter hyperintensities, either in periventricular or in deep white matter. In contrast, Babiarz et al.22 did not find any correlation between IAC and white-matter ischemia.
In a subpopulation of 2,414 nondemented people in the Rotterdam Study, investigators found a larger IICA calcification volume to be associated with worse cognitive performance, which might be attributable to a smaller brain tissue volume and worse microstructural integrity of the white matter in individuals with heavier atherosclerotic burdens.56 A subsequence longitudinal study reconfirmed that a larger IICA calcification volume was related to a higher risk of cognitive decline.57 Multicenter studies are required to verify the role of IAC in predicting cognitive impairment and dementia.
To summarize, the findings from clinical studies suggest that IAC—as an indicator of intracranial atherosclerosis—is associated with ischemic stroke, different radiological brain changes including white-matter disease or microbleeds, and also cognitive impairment. More evidence is needed from well-designed studies to verify the predictive values of IAC in multiple vascular events and to clarify the related underlying pathophysiology.
The prevalence of IAC is high worldwide. As a vascular lesion changes over time, aging is a definite risk factor for IAC. In addition, sex and common cardiovascular risk factors also contribute to the occurrence and progression of IAC. IAC within the cerebral vasculature is mostly observed in the IICA, followed by the vertebral artery, basilar artery, and middle cerebral artery. Although IAC is frequently observed, diverse methods are currently used to assess its severity, and consistent criteria for comparing findings from different centers is lacking. A well-designed computer-assisted objective software tool may be more useful than qualitative visual grading methods for measuring the severity of IAC both quantitatively and reproducibly. An accurate and consistent quantitative measurement method, which will hopefully be accepted by different researchers worldwide, needs to be validated and developed in larger clinical studies.
As an indicator of atherosclerosis, IAC may induce hemodynamic changes because of in-site luminal stenosis and subsequent increased arterial stiffness. However, its association with plaque instability is still controversial. There is accumulating evidence from cross-sectional or longitudinal studies that IAC is associated with ischemic stroke, imaging-verified brain changes (white-matter disease or microbleeds), and cognitive impairment in both Asians and Whites. More well-designed clinical studies that combine multiple imaging modalities are needed to explore the predictive values of IAC in vascular events and the underlying pathophysiological mechanisms.
Figures and Tables
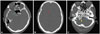 | Fig. 1Examples of different degrees of intracranial artery calcification on a noncontrast CT image. According to Babiarz's visual grading scales, continuous calcifications were graded as follows. A: RICA, 3 for extent and 2 for thickness. LICA, 4 for extent and 3 for thickness. B: RACA, 2 for extent and 2 for thickness. C: RVA, 3 for extent and 3 for thickness. LVA, 2 for extent and 2 for thickness. LICA: left internal carotid artery, LVA: left vertebral artery, RACA: right anterior cerebral artery, RICA: right internal carotid artery, RVA: right vertebral artery. |
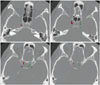 | Fig. 2Color-overlay images showing semiautomatic segmentations of the bilateral intracranial carotid artery calcification by commercial software in our center (red for calcification in RICA and green for calcification in LICA). LICA: left internal carotid artery, RICA: right internal carotid artery. |
Table 1
Risk factors for IAC identified in previous studies

| Study authors and year | Subjects | Sample size | Risk factors for IAC |
|---|---|---|---|
| Bos et al., 201217 | Population-based participants receiving head CT scans | 2,495 | Aging, diabetes, hypercholesterolemia, and history of cardiovascular disease (males: excessive alcohol intake; females: hypertension) |
| Power et al., 201120 | Hemodialysis patients receiving head CT scans | 490 | Aging, increasing duration of hemodialysis, diabetes, and established cardiac and peripheral arterial diseases |
| Bugnicourt et al., 200919 | Patients with ischemic stroke receiving head CT scans | 340 | Aging, severe atherosclerosis, and the presence of chronic kidney disease |
| de Weert et al., 200916 | Patients receiving multidetector CTA of the carotid arteries | 406 | Aging, male sex, and cardiovascular risk factors (smoking, hypertension, hypercholesterolemia, diabetes, history of cardiac disease, and history of cerebrovascular disease) |
| Chen et al., 200613 | Patients referred for head CT scans | 490 | Aging, history of ischemic stroke, and white blood cell count |
| Sohn et al., 200415 | Patients with ischemic stroke receiving head CT scans | 57 | Aging and hypertension |
| Ptak et al., 200358 | Patients referred for head CT scans | 295 | Male sex, hypertension, and hypercholesterolemia |
Table 2
Current methods for calcification measurement

Acknowledgements
This work was supported by Health and Medical Research Fund (HMRF, Project No. 11120161), Shenzhen Science and Technology Innovation Committee (SZSTI, Project No. JC20140606164105360), and the National Natural Science Foundation of China (NSFC, Project No. 81371297).
References
1. Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009; 4:1892–1900.

2. Vos A, Van Hecke W, Spliet WG, Goldschmeding R, Isgum I, Kockelkoren R, et al. Predominance of nonatherosclerotic internal elastic lamina calcification in the intracranial internal carotid artery. Stroke. 2016; 47:221–223.

3. Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014; 35:1515–1525.

4. Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004; 24:1161–1170.
5. Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995; 91:2488–2496.
6. Boström K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993; 91:1800–1809.

7. Hirota S, Imakita M, Kohri K, Ito A, Morii E, Adachi S, et al. Expression of osteopontin messenger RNA by macrophages in atherosclerotic plaques. A possible association with calcification. Am J Pathol. 1993; 143:1003–1008.
8. Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001; 21:1998–2003.

9. O'Rourke RA, Brundage BH, Froelicher VF, Greenland P, Grundy SM, Hachamovitch R, et al. American College of Cardiology/American Heart Association Expert Consensus document on electronbeam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation. 2000; 102:126–140.
10. Ratinov G. Extradural intracranial portion of carotid artery; a clinicopathologic study. Arch Neurol. 1964; 10:66–73.
11. Fisher CM, Gore I, Okabe N, White PD. Calcification of the carotid siphon. Circulation. 1965; 32:538–548.

12. Boström K, Hassler O. Radiological study of arterial calcification. 2. Intracranial arteries. Neurology. 1965; 15:1168–1172.

13. Chen XY, Lam WW, Ng HK, Fan YH, Wong KS. The frequency and determinants of calcification in intracranial arteries in Chinese patients who underwent computed tomography examinations. Cerebrovasc Dis. 2006; 21:91–97.

14. Mak HK, Wong CW, Yau KK, Wong WM, Gu J, Khong PL, et al. Computed tomography evaluation of intracranial atherosclerosis in Chinese patients with transient ischemic attack or minor ischemic stroke--its distribution and association with vascular risk factors. J Stroke Cerebrovasc Dis. 2009; 18:158–163.

15. Sohn YH, Cheon HY, Jeon P, Kang SY. Clinical implication of cerebral artery calcification on brain CT. Cerebrovasc Dis. 2004; 18:332–337.

16. de Weert TT, Cakir H, Rozie S, Cretier S, Meijering E, Dippel DW, et al. Intracranial internal carotid artery calcifications: association with vascular risk factors and ischemic cerebrovascular disease. AJNR Am J Neuroradiol. 2009; 30:177–184.

17. Bos D, van der Rijk MJ, Geeraedts TE, Hofman A, Krestin GP, Witteman JC, et al. Intracranial carotid artery atherosclerosis: prevalence and risk factors in the general population. Stroke. 2012; 43:1878–1884.
18. Gusbeth-Tatomir P, Covic A. Causes and consequences of increased arterial stiffness in chronic kidney disease patients. Kidney Blood Press Res. 2007; 30:97–107.

19. Bugnicourt JM, Chillon JM, Massy ZA, Canaple S, Lamy C, Deramond H, et al. High prevalence of intracranial artery calcification in stroke patients with CKD: a retrospective study. Clin J Am Soc Nephrol. 2009; 4:284–290.

20. Power A, Chan K, Haydar A, Hamady M, Cairns T, Taube D, et al. Intracranial arterial calcification is highly prevalent in hemodialysis patients but does not associate with acute ischemic stroke. Hemodial Int. 2011; 15:256–263.

21. Woodcock RJ Jr, Goldstein JH, Kallmes DF, Cloft HJ, Phillips CD. Angiographic correlation of CT calcification in the carotid siphon. AJNR Am J Neuroradiol. 1999; 20:495–499.
22. Babiarz LS, Yousem DM, Wasserman BA, Wu C, Bilker W, Beauchamp NJ Jr. Cavernous carotid artery calcification and white matter ischemia. AJNR Am J Neuroradiol. 2003; 24:872–877.
23. Babiarz LS, Yousem DM, Bilker W, Wasserman BA. Middle cerebral artery infarction: relationship of cavernous carotid artery calcification. AJNR Am J Neuroradiol. 2005; 26:1505–1511.
24. Taoka T, Iwasaki S, Nakagawa H, Sakamoto M, Fukusumi A, Takayama K, et al. Evaluation of arteriosclerotic changes in the intracranial carotid artery using the calcium score obtained on plain cranial computed tomography scan: correlation with angiographic changes and clinical outcome. J Comput Assist Tomogr. 2006; 30:624–628.

25. Erbay S, Han R, Baccei S, Krakov W, Zou KH, Bhadelia R, et al. Intracranial carotid artery calcification on head CT and its association with ischemic changes on brain MRI in patients presenting with stroke-like symptoms: retrospective analysis. Neuroradiology. 2007; 49:27–33.

26. Chung PW, Park KY, Moon HS, Kim YB, Youn YC, Byun JS, et al. Intracranial internal carotid artery calcification: a representative for cerebral artery calcification and association with white matter hyperintensities. Cerebrovasc Dis. 2010; 30:65–71.

27. Bleeker L, Marquering HA, van den Berg R, Nederkoorn PJ, Majoie CB. Semi-automatic quantitative measurements of intracranial internal carotid artery stenosis and calcification using CT angiography. Neuroradiology. 2012; 54:919–927.

28. Ahn SS, Nam HS, Heo JH, Kim YD, Lee SK, Han K, et al. Quantification of intracranial internal carotid artery calcification on brain unenhanced CT: evaluation of its feasibility and assessment of the reliability of visual grading scales. Eur Radiol. 2013; 23:20–27.

29. Marquering HA, Nederkoorn PJ, Bleeker L, van den Berg R, Majoie CB. Intracranial carotid artery disease in patients with recent neurological symptoms: high prevalence on CTA. Neuroradiology. 2013; 55:179–185.

30. Yilmaz A, Akpinar E, Topcuoglu MA, Arsava EM. Clinical and imaging features associated with intracranial internal carotid artery calcifications in patients with ischemic stroke. Neuroradiology. 2015; 57:501–506.

31. Katada K, Kanno T, Sano H, Shinomiya Y, Koga S. Calcification of the vertebral artery. AJNR Am J Neuroradiol. 1983; 4:450–453.

32. Pikija S, Magdič J, Hojs-Fabjan T. Calcifications of vertebrobasilar arteries on CT: detailed distribution and relation to risk factors in 245 ischemic stroke patients. Biomed Res Int. 2013; 2013:918970.

33. Higgins CL, Marvel SA, Morrisett JD. Quantification of calcification in atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2005; 25:1567–1576.

34. Schoenhagen P, Halliburton SS, Stillman AE, Kuzmiak SA, Nissen SE, Tuzcu EM, et al. Noninvasive imaging of coronary arteries: current and future role of multi-detector row CT. Radiology. 2004; 232:7–17.

35. Koton S, Tashlykov V, Schwammenthal Y, Molshatzki N, Merzeliak O, Tsabari R, et al. Cerebral artery calcification in patients with acute cerebrovascular diseases: determinants and long-term clinical outcome. Eur J Neurol. 2012; 19:739–745.

36. Bugnicourt JM, Chillon JM, Tribouilloy C, Canaple S, Lamy C, Massy ZA, et al. Relation between intracranial artery calcifications and aortic atherosclerosis in ischemic stroke patients. J Neurol. 2010; 257:1338–1343.

37. Bugnicourt JM, Leclercq C, Chillon JM, Diouf M, Deramond H, Canaple S, et al. Presence of intracranial artery calcification is associated with mortality and vascular events in patients with ischemic stroke after hospital discharge: a cohort study. Stroke. 2011; 42:3447–3453.

38. Coutinho T, Turner ST, Kullo IJ. Aortic pulse wave velocity is associated with measures of subclinical target organ damage. JACC Cardiovasc Imaging. 2011; 4:754–761.

39. Zhang J, Li Y, Wang Y, Niu W, Zhang Y, Gao P, et al. Arterial stiffness and asymptomatic intracranial large arterial stenosis and calcification in hypertensive chinese. Am J Hypertens. 2011; 24:304–309.

40. Park KY, Kim YB, Moon HS, Suh BC, Chung PW. Association between cerebral arterial calcification and brachial-ankle pulse wave velocity in patients with acute ischemic stroke. Eur Neurol. 2009; 61:364–370.

41. Kassab MY, Gupta R, Majid A, Farooq MU, Giles BP, Johnson MD, et al. Extent of intra-arterial calcification on head CT is predictive of the degree of intracranial atherosclerosis on digital subtraction angiography. Cerebrovasc Dis. 2009; 28:45–48.

42. Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001; 103:1051–1056.

43. Kitagawa T, Yamamoto H, Horiguchi J, Ohhashi N, Tadehara F, Shokawa T, et al. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging. 2009; 2:153–160.

44. Nicholls SJ, Tuzcu EM, Wolski K, Sipahi I, Schoenhagen P, Crowe T, et al. Coronary artery calcification and changes in atheroma burden in response to established medical therapies. J Am Coll Cardiol. 2007; 49:263–270.

45. Otsuka F, Finn AV, Virmani R. Do vulnerable and ruptured plaques hide in heavily calcified arteries? Atherosclerosis. 2013; 229:34–37.

46. Shaalan WE, Cheng H, Gewertz B, McKinsey JF, Schwartz LB, Katz D, et al. Degree of carotid plaque calcification in relation to symptomatic outcome and plaque inflammation. J Vasc Surg. 2004; 40:262–269.

47. Chen XY, Wong KS, Lam WW, Zhao HL, Ng HK. Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc Dis. 2008; 25:74–80.

48. Pletcher MJ, Sibley CT, Pignone M, Vittinghoff E, Greenland P. Interpretation of the coronary artery calcium score in combination with conventional cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2013; 128:1076–1084.

50. Chen XY, Lam WW, Ng HK, Fan YH, Wong KS. Intracranial artery calcification: a newly identified risk factor of ischemic stroke. J Neuroimaging. 2007; 17:300–303.

51. Ovesen C, Abild A, Christensen AF, Rosenbaum S, Hansen CK, Havsteen I, et al. Prevalence and long-term clinical significance of intracranial atherosclerosis after ischaemic stroke or transient ischaemic attack: a cohort study. BMJ Open. 2013; 3:e003724.

52. Bos D, Portegies ML, van der Lugt A, Bos MJ, Koudstaal PJ, Hofman A, et al. Intracranial carotid artery atherosclerosis and the risk of stroke in whites: the Rotterdam Study. JAMA Neurol. 2014; 71:405–411.

53. van Dijk AC, Fonville S, Zadi T, van Hattem AM, Saiedie G, Koudstaal PJ, et al. Association between arterial calcifications and nonlacunar and lacunar ischemic strokes. Stroke. 2014; 45:728–733.

54. Hong NR, Seo HS, Lee YH, Kim JH, Seol HY, Lee NJ, et al. The correlation between carotid siphon calcification and lacunar infarction. Neuroradiology. 2011; 53:643–649.

55. Chung PW, Park KY, Kim JM, Shin DW, Ha SY. Carotid artery calcification is associated with deep cerebral microbleeds. Eur Neurol. 2014; 72:60–63.

56. Bos D, Vernooij MW, Elias-Smale SE, Verhaaren BF, Vrooman HA, Hofman A, et al. Atherosclerotic calcification relates to cognitive function and to brain changes on magnetic resonance imaging. Alzheimers Dement. 2012; 8:5 Suppl. S104–S111.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download