Dear Editor,
Acute disseminated encephalomyelitis (ADEM) is a monophasic acute nonvasculitic inflammatory demyelinating disorder of the central nervous system that is characterized by diffuse neurologic signs and symptoms coupled with evidence of multifocal demyelination lesions on neuroimaging.1 The onset of ADEM usually follows a viral infection or immunization after a mean period of 7–14 days. Neuroinflammation is well established as a key secondary injury mechanism after traumatic brain injury (TBI), and it has been long considered to contribute to the damage sustained following TBI.2 Early diagnosis and prompt treatment of ADEM are likely to reduce morbidity.3
A 4-year-old girl banged the back of her head and neck while bathing. She lost conscious for few seconds, and was agitated during the postconcussion period. After 72 hours her score on the Glasgow Coma Scale (GCS) was 8 (eye opening=3, verbal=4, and motor=1), and she had bilateral reactive pupils, generalized symmetrical hypotonia, intact sensation, and intact knee and ankle reflexes. The plantar response was bilateral flexor, and she reported backache. A CSF examination showed pleocytosis (70 cells/µL) with mild elevation of protein (50 mg/dL), negative CSF oligoclonal bands, and negative serum neuromyelitis optica-immunoglobulin G antibodies (ELISA/dilution 4×). MRI of the cervical spine and brain confirmed a diagnosis of ADEM (Fig. 1).
She showed a marked improvement after pulse steroid therapy with methylprednisolone sodium succinate (Solu-Medrol®, Pfizer Inc., Belgium) at 30 mg/kg daily for 3 days. She was continued on oral prednisolone that was subsequently tapered over a 3-week period. A 3-week follow-up MRI examination showed regression of the disease process in terms of size, number, and mass effect of lesions. After 2 months she exhibited a complete clinical recovery, and normal neurologic findings.
Posttraumatic ADEM is rarely reported. An abnormally low GCS score at 24 hours or longer after head trauma is associated with an unfavorable prognosis.4 In the present case the GCS score remained low at 3 days posttrauma.
In our case brain demyelination occurred 72 hours after TBI. An animal study found that neuroinflammation plays a key role in the pathophysiology of brain damage following TBI. Biphasic breakdown of the blood-brain barrier after TBI has been reported to first appear immediately after the primary insult, with the permeability peaking within a few hours and then subsequently improving. Delayed injury cascades typically peak at 3–7 days after TBI, and lasts from days to years.5 Persistent inflammation and ongoing white-matter degeneration lasting many years after a single TBI have been reported in humans. PET imaging in humans using a ligand that binds to activated microglia revealed increased microglial activation for up to 17 years after TBI.6 Multiple or single extensive lesions on MRI in ADEM syndrome may be associated with disability.7 This contrasts with our case, since she recovered completely despite having multiple lesions. It is unclear whether the complete recovery in our case was due to the underlying etiology or to the early application of treatment. A previous study found a mortality rate of 20% with a high incidence of neurologic sequelae in patients who survived postmeasles ADEM.8
Figures and Tables
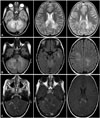
Fig. 1
Axial T2-weighted (A) and FLAIR (B) MRI images of the brain, showing bilateral cerebral and cerebellar ill-defined patchy areas of signal abnormalities in the middle cerebellar peduncles, left temporal periventricular region, splenium of the corpus callosum, and centrum semiovale white matter. In axial postcontrast T1-weighted MRI with magnetization transfer contrast (C), the lesions showed patchy enhancement (arrow).
References
1. Dale RC, Brilot F, Banwell B. Pediatric central nervous system inflammatory demyelination: acute disseminated encephalomyelitis, clinically isolated syndromes, neuromyelitis optica, and multiple sclerosis. Curr Opin Neurol. 2009; 22:233–240.

2. Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012; 26:1191–1201.

3. Gupte G, Stonehouse M, Wassmer E, Coad NA, Whitehouse WP. Acute disseminated encephalomyelitis: a review of 18 cases in childhood. J Paediatr Child Health. 2003; 39:336–342.

4. Lee SY, Kim SS, Kim CH, Park SW, Park JH, Yeo M. Prediction of outcome after traumatic brain injury using clinical and neuroimaging variables. J Clin Neurol. 2012; 8:224–229.

5. Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010; 6:393–403.

6. Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011; 70:374–383.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download