Abstract
Helicobacter pylori (HP) is a common infection of the gastrointestinal system that is usually related to peptic ulcers. However, recent studies have revealed relationships between HP and many other diseases. Although the exact mechanism is unknown, HP can prevent the absorption of certain drugs. A high prevalence of HP has been found in patients with Parkinson's disease, and this bacterium causes motor fluctuations by affecting the absorption of levodopa, which is the main drug used to treat Parkinson's disease. Eradicating HP from patients with Parkinson's disease by applying antibiotic treatment will increase the absorption of levodopa and decrease their motor fluctuations.
Parkinson's disease (PD) was first described by James Parkinson in 1817 with the name of 'shaking palsy'.1 PD is a progressive neurodegenerative disease that occurs due to the loss of dopaminergic neurons in the substantia nigra pars compacta. PD is a complex and multisystem disease of unknown etiology.2 However, recent studies have found that infection plays a role in the etiology of PD, such as by bacterial overgrowth and involving cytomegalovirus, Epstein Barr virus, herpes simplex virus type-1 and Helicobacter pylori (HP) in the small intestine.34 The most-common symptoms are related to the motor system (bradykinesia/hypokinesia, rigidity, tremor and postural abnormality), with also non-motor symptoms such as constipation, pain, genitourinary problems, sleep disorders, depression, autonomic dysfunction, cognitive abnormalities, dementia and gastrointestinal dysfunction.2567
HP is a Gram-negative bacterium found on the luminal surface of the gastric epithelium, and it was first isolated by Warren and Marshall8 in 1983. HP induces chronic inflammation of the underlying mucosa, with the infection usually being contracted in the first years of life and tending to persist indefinitely unless treated.8 It was initially found to cause gastritis, peptic ulcer and gastric cancer. However, subsequent studies have revealed that it is also related to diseases of other systems such as hematological disorders (iron-deficiency anemia and idiopathic thrombocytopenic purpura),9 cardiovascular diseases (ischemic heart disease), neurological diseases (stroke, PD, and Alzheimer's disease), obesity, and dermatological diseases.10
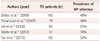
A few studies have found the prevalence of HP infection to be high in PD patient. Dobbs et al.11 found that the urea breath test was positive in 48% of 105 PD patient. while Pierantozzi et al.,12 Lee et al.,13 Dobbs et al.,14 and Tan et al.,15 obtained positive HP results in 36%, 53%, 70%, and 32% of 79, 65, 51 and of 102 PD patients, respectively (Table 1).
The relationship between PD and gastric ulcer was first reported in 1960. An increased prevalence of gastric ulcer in PD patients was first described as an independent component of the disease, resulting in gastrointestinal symptoms being considered one of the symptoms experienced by all PD patients. This means that gastritis as hypokinesia might also be seen in PD patients. Researchers found that there was a relationship between PD and HP, and that HP might actually cause PD.11181920
One proposed hypothesis was that HP shows a neurotoxic effect by increasing cholesterol glucosides, with HP causing PD by degenerating dopaminergic neurons in the brain.20
A second proposed hypothesis is that when HP infection is not controlled by the immune system or HP is not eradicated, HP causes the development of PD by damaging dopaminergic cells in the brain.21 It was stated that HP might lead to the pathogenesis of PD by causing apoptosis of nerve cells after passing the blood-brain barrier after oral ingestion, nasal odor inhalation, or via circulating monocytes.22
The primary pharmacological agent used in PD patients to replace dopamine is levodopa.23 HP not only causes the development of PD but also leads to motor fluctuations in PD patients by affecting the absorption of levodopa.24 HP infection prevents the absorption of levodopa, thyroxine and delavirdine, with the absorption of levodopa in PD patients being observed to increase by 21–54% following HP-eradication.25 HP infection is thought to affect drug absorption via its potential effects on intragastric pH.2526
It was further found that successful eradication of HP in PD patients decreased motor fluctuations by affecting the bioavailability of levodopa. These finding together indicate the potential importance of HP eradication in PD patients.27
Lee et al.13 investigated the effects of HP on the clinical response to levodopa and whether motor fluctuations in PD patients could be decreased by HP eradication. Those authors investigated the presence of HP in 65 PD patients using the urea breath test. HP was detected in 35 of these patients, and so the response to levodopa and clinical features were compared between these two groups. The onset of the effect of levodopa, the duration of drug action ('on-time'), and the motor movements as measured by the Unified Parkinson's Disease Rating Scale (UPDRS) were evaluated in both groups. There was no difference between the groups in terms of age, duration of disease, scores on the Hoehn-Yahr scale or UPDRS-III, daily dose of levodopa, or frequency of dyskinesia. In PD patients with HP infection, the onset time of levodopa was longer, and the 'on-time' was shorter compared to PD patients who did not have HP infection. These data suggest that HP prevents the absorption of levodopa in PD patient. After administering antibiotic treatment to PD patients to eradicate HP, the 'onset' time decreased and the 'on-time' increased when compared to the pretreatment values. That study concluded that motor fluctuations might be decreased in PD patients by eradicating HP.
Tan et al.15 found that 32.4% of 102 PD patients were infected with HP. These infected PD patients were older (age, 68.4±7.3 years, mean±SD) and exhibited worse motor function (UPDRS-III score, 34.0±13.0 vs. 27.3±10.0, p=0.04; pegboard test, 6.4±3.3 pins vs. 8.0±2.7 pins, p=0.04; and 'timed-gait' test, 25.1±25.4 s vs. 15.5±7.6 s, p=0.08). That study revealed important effects of HP infection on UPDRS-III and 'timed-gait' tests of PD patients; it was found that HP status and its relationship with these motor results varied according to age.
Pierantozzi et al.28 applied HP eradication to 17 patients and administered a common antioxidant treatment to another 17 patients among 34 PD patients with HP infection. They found that the absorption of levodopa was significantly increased in the HP-eradication group relative to the placebo group. That study found that HP affected the absorption of levodopa, which caused motor fluctuations by decreasing the 'on-time'.
The study of Rahne et al.29 involved 75 patients who had been diagnosed with PD at least 4 years previously, and evaluated symptom fluctuations by using UPDRS-IV and the 9-items Wearing-Off-Questionnaire scale. HP infection was detected in 20 (27%) patients using the urea breath test, and levodopa doses of PD patients with HP infection were higher. In HP-positive PD patients enrolled in the study, 'wearing off' and sleep disorders were significantly less common. Symptom fluctuations also decreased in HP-positive PD patients. That study showed that HP infection might change the absorption of levodopa in the gastrointestinal tract.
Dobbs et al.30 reported that 21 of 66 PD patients had previously received HP treatment, and that 13 had HP. The urea breath tests performed in that study detected HP positivity in 40 patients. They found that HP eradication improved tremor, rigidity, hypokinesia and walking ability. Other antimicrobial treatments were found to be insufficient for treating HP, and resulted in worsening rigidity and tremor in the patients. That study demonstrated that HP should be eradicated by applying proper antibiotic treatment.
Bjarnason et al.31 conducted a randomized, double blind and placebo controlled study to investigate the relationship between HP infection and idiopathic PD. The patients were followed for 1 year to monitor the success of HP eradication. Those authors concluded that HP infection might cause idiopathic PD. Stride length and rigidity were found to be worse in HP positive PD patients. Successful HP eradication decreased the symptoms in patients with idiopathic PD, and HP infection was found to affect the absorption of levodopa.
In a randomized, placebo controlled, and parallel group study, Dobbs et al.32 found that the mean stride length was 3 mm (range, -47 to 52 mm) in the placebo group (n=16) and 75 mm (range, 41 to 110 mm) in the control group (n=12, HP eradicated group) (p=0.01). the mean extension rigidity was -1×10-3 Nm (range, -141×10-3 to 143×10-3 Nm) in the placebo group and 238×10-3 Nm (range, 115×10-3 to 361×10-3 Nm) in the control group (p=0.01). HP was not eradicated in four patients in whom stride lengths were found to be shorter.
Hashim et al.33 obtained positive results in the urea breath test in 27 (32.9%) of 82 PD patients. UPDRS (p=0.005), with 39-items Parkinson's Disease Questionnaire (PDQ39) (p< 0.0001) scores significantly worse for HP-positive patients than for HP-negative PD patients. The levodopa 'onset' time shortened and the 'on-time' increased in the patients following 14 weeks of HP-eradication treatment, while the UPDRS and PDQ39 scores also improved significantly.
Pierantozzi et al.28 detected fluctuations in levodopa absorption in six HP-positive PD patients but not in HP-negative patients, and the UPDRS scores of the patients decreased after HP eradication.
Niehues and Hensel26 investigated the in-vitro effect of HP on levodopa, and found that HP caused a significant decrease in the levodopa concentration, and therefore might also be able to change the clinical status of PD patients.
Narożańska et al.34 detected HP positivity in 18 of 37 PD patients, but found no significant difference between HP-positive and HP-negative patients in terms of scores on the UPDRS and Hoehn-Yahr scales.
The present study was part an ongoing study of the effects of HP eradication to PD patients that has a double-blind, randomized, placebo-controlled trial design involving a relatively large cohort of patients. The trial started in December 2013 and the results are still not disclosed. The results of that larger study could provide us with more-accurate results.35
The prevalence of HP infection is high among PD patients. Various studies have revealed that HP causes motor fluctuations in PD patients by affecting the absorption of levodopa. Therefore, PD patients should be evaluated for the presence of HP infection and eradication treatment can be applied in cases where HP positivity is detected. The relationship between HP and PD should be further clarified by more-comprehensive studies in the future.
Figures and Tables
References
1. Memiş S. Parkinson hastalığı ve bakım. In : Durna Z, editor. Kronik Hastalıklar ve Bakım. İstanbul: Nobel Tıp Kitabevi;2011. p. 277.
2. Barnum CJ, Tansey MG. Neuroinflammation and non-motor symptoms: the dark passenger of Parkinson's disease? Curr Neurol Neurosci Rep. 2012; 12:350–358.

3. Bu XL, Wang X, Xiang Y, Shen LL, Wang QH, Liu YH, et al. The association between infectious burden and Parkinson's disease: a case-control study. Parkinsonism Relat Disord. 2015; 21:877–881.

4. Tan AH, Mahadeva S, Thalha AM, Gibson PR, Kiew CK, Yeat CM, et al. Small intestinal bacterial overgrowth in Parkinson's disease. Parkinsonism Relat Disord. 2014; 20:535–540.

5. Chaudhuri KR, Healy DG, Schapira AH. National Institute for Clinical Excellence. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 2006; 5:235–245.

6. Simuni T, Sethi K. Nonmotor manifestations of Parkinson's disease. Ann Neurol. 2008; 64:Suppl 2. S65–S80.

7. Lim SY, Lang AE. The nonmotor symptoms of Parkinson's disease--an overview. Mov Disord. 2010; 25:Suppl 1. S123–S130.

8. McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010; 362:1597–1604.
9. Franceschi F, Gasbarrini A. Helicobacter pylori and extragastric diseases. Best Pract Res Clin Gastroenterol. 2007; 21:325–334.

10. Tan HJ, Goh KL. Extragastrointestinal manifestations of Helicobacter pylori infection: facts or myth? A critical review. J Dig Dis. 2012; 13:342–349.

11. Dobbs RJ, Charlett A, Dobbs SM, Weller C, Peterson DW. Parkinsonism: differential age-trend in Helicobacter pylori antibody. Aliment Pharmacol Ther. 2000; 14:1199–1205.

12. Pierantozzi M, Pietroiusti A, Brusa L, Galati S, Stefani A, Lunardi G, et al. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology. 2006; 66:1824–1829.

13. Lee WY, Yoon WT, Shin HY, Jeon SH, Rhee PL. Helicobacter pylori infection and motor fluctuations in patients with Parkinson's disease. Mov Disord. 2008; 23:1696–1700.

14. Dobbs RJ, Charlett A, Dobbs SM, Weller C, A Ibrahim MA, Iguodala O, et al. Leukocyte-subset counts in idiopathic parkinsonism provide clues to a pathogenic pathway involving small intestinal bacterial overgrowth. A surveillance study. Gut Pathog. 2012; 4:12.

15. Tan AH, Mahadeva S, Marras C, Thalha AM, Kiew CK, Yeat CM, et al. Helicobacter pylori infection is associated with worse severity of Parkinson's disease. Parkinsonism Relat Disord. 2015; 21:221–225.

16. Weller C, Charlett A, Oxlade NL, Dobbs SM, Dobbs RJ, Peterson DW, et al. Role of chronic infection and inflammation in the gastrointestinal tract in the etiology and pathogenesis of idiopathic parkinsonism. Part 3: predicted probability and gradients of severity of idiopathic parkinsonism based on H. pylori antibody profile. Helicobacter. 2005; 10:288–297.

17. Charlett A, Dobbs RJ, Dobbs SM, Weller C, Brady P, Peterson DW. Parkinsonism: siblings share Helicobacter pylori seropositivity and facets of syndrome. Acta Neurol Scand. 1999; 99:26–35.

18. Blaecher C, Smet A, Flahou B, Pasmans F, Ducatelle R, Taylor D, et al. Significantly higher frequency of Helicobacter suis in patients with idiopathic parkinsonism than in control patients. Aliment Pharmacol Ther. 2013; 38:1347–1353.

19. Dobbs SM, Dobbs RJ, Weller C, Charlett A. Link between Helicobacter pylori infection and idiopathic parkinsonism. Med Hypotheses. 2000; 55:93–98.

20. Schulz JD, Hawkes EL, Shaw CA. Cycad toxins, Helicobacter pylori and parkinsonism: cholesterol glucosides as the common denomenator. Med Hypotheses. 2006; 66:1222–1226.

21. Dobbs RJ, Dobbs SM, Weller C, Charlett A, Bjarnason IT, Curry A, et al. Helicobacter hypothesis for idiopathic parkinsonism: before and beyond. Helicobacter. 2008; 13:309–322.
22. Kountouras J, Zavos C, Polyzos SA, Deretzi G, Vardaka E, Giartza-Taxidou E, et al. Helicobacter pylori infection and Parkinson's disease: apoptosis as an underlying common contributor. Eur J Neurol. 2012; 19:e56.

23. Fasano A, Bove F, Gabrielli M, Ragazzoni E, Fortuna S, Tortora A, et al. Liquid melevodopa versus standard levodopa in patients with Parkinson disease and small intestinal bacterial overgrowth. Clin Neuropharmacol. 2014; 37:91–95.

24. Pfeiffer R. Beyond here be dragons: SIBO in Parkinson's disease. Mov Disord. 2013; 28:1764–1765.

25. Lahner E, Annibale B, Delle Fave G. Systematic review: Helicobacter pylori infection and impaired drug absorption. Aliment Pharmacol Ther. 2009; 29:379–386.

26. Niehues M, Hensel A. In-vitro interaction of L-dopa with bacterial adhesins of Helicobacter pylori: an explanation for clinicial differences in bioavailability? J Pharm Pharmacol. 2009; 61:1303–1307.

27. Lyte M. Microbial endocrinology as a basis for improved L-DOPA bioavailability in Parkinson's patients treated for Helicobacter pylori. Med Hypotheses. 2010; 74:895–897.

28. Pierantozzi M, Pietroiusti A, Sancesario G, Lunardi G, Fedele E, Giacomini P, et al. Reduced L-dopa absorption and increased clinical fluctuations in Helicobacter pylori-infected Parkinson's disease patients. Neurol Sci. 2001; 22:89–91.

29. Rahne KE, Tagesson C, Nyholm D. Motor fluctuations and Helicobacter pylori in Parkinson's disease. J Neurol. 2013; 260:2974–2980.

30. Dobbs SM, Charlett A, Dobbs RJ, Weller C, Iguodala O, Smee C, et al. Antimicrobial surveillance in idiopathic parkinsonism: indication-specific improvement in hypokinesia following Helicobacter pylori eradication and non-specific effect of antimicrobials for other indications in worsening rigidity. Helicobacter. 2013; 18:187–196.

31. Bjarnason IT, Charlett A, Dobbs RJ, Dobbs SM, Ibrahim MA, Kerwin RW, et al. Role of chronic infection and inflammation in the gastrointestinal tract in the etiology and pathogenesis of idiopathic parkinsonism. Part 2: response of facets of clinical idiopathic parkinsonism to Helicobacter pylori eradication. A randomized, double-blind, placebo-controlled efficacy study. Helicobacter. 2005; 10:276–287.

32. Dobbs SM, Dobbs RJ, Weller C, Charlett A, Bjarnason IT, Lawson AJ, et al. Differential effect of Helicobacter pylori eradication on time-trends in brady/hypokinesia and rigidity in idiopathic parkinsonism. Helicobacter. 2010; 15:279–294.

33. Hashim H, Azmin S, Razlan H, Yahya NW, Tan HJ, Manaf MR, et al. Eradication of Helicobacter pylori infection improves levodopa action, clinical symptoms and quality of life in patients with Parkinson's disease. PLoS One. 2014; 9:e112330.

34. Narożańska E, Białecka M, Adamiak-Giera U, Gawrońska-Szklarz B, Sołtan W, Schinwelski M, et al. Pharmacokinetics of levodopa in patients with Parkinson disease and motor fluctuations depending on the presence of Helicobacter pylori infection. Clin Neuropharmacol. 2014; 37:96–99.

35. ClinicalTrials.gov (US). Helicobacter pylori eradication in Parkinson's disease: a double-blind randomised placebo controlled trial [Internet]. Kuala Lumpur: University of Malaya;2014. cited 2015 Sep 16. Available from: https://clinicaltrials.gov/show/NCT02108704.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download