Abstract
Background and Purpose
The aim of this study was to determine the changes in diffusion-tensor images associated with medication-related impulse control disorder (ICD) in Parkinson's disease (PD) patients undergoing chronic dopamine-replacement therapy.
Methods
Nineteen PD patients, comprising 10 with ICD (PD-ICD) and 9 without ICD (PD-nonICD), and 18 age-matched healthy controls (HCs) with no cognitive or other psychiatric disorders were analyzed. All subjects underwent 3-T magnetic resonance diffusion-tensor imaging. For all PD patients, clinical data on PD duration, antiparkinsonian medication dosages, Unified Parkinson's Disease Rating Scale and Mini-Mental State Examination were collected. Whole-brain voxel-based measures of fractional anisotropy (FA) and mean diffusivity (MD) were analyzed.
Results
In comparison with HCs, the PD-nonICD subjects had low FA at the bilateral orbitofrontal areas. While the PD-ICD subjects exhibited no such difference, their FA was significantly elevated at the anterior corpus callosum. Analysis of FA between the two PD groups revealed that FA in the anterior corpus callosum, right internal capsule posterior limbs, right posterior cingulum, and right thalamic radiations were significantly higher (corrected p<0.05) in the PD-ICD than in the PD-nonICD patients. MD did not differ between the PD-ICD and PD-nonICD groups in any brain regions.
Conclusions
The PD-ICD patients appear to have relatively preserved white-matter integrity in the regions involved in reward-related behaviors compared to PD-nonICD patients. Further investigation is required to determine whether the difference in FA between PD-ICD and PD-nonICD patients reflects microstructural differences in the pathological progression of PD or is secondary to ICD.
Impulse control disorder (ICD) occurs more frequently in patients with Parkinson's disease (PD) than in the general population.1 Large cross-sectional studies have found that the prevalence of ICD ranges from 10% to 15% in patients taking dopaminergic drugs.1,2 Common ICD behaviors in PD are pathological gambling, compulsive shopping, hypersexuality, and binge eating, and more than 25% of PD patients with ICD exhibit two or more of these behaviors simultaneously.1,2
Dopaminergic drugs improve parkinsonian motor symptoms; however, they can also cause ICD, with a reported prevalence of more than 10% in northeast Asia.2 The finding of differences between patients in the susceptibility to ICD onset2 suggests that there are other factors involved in pathogenesis of ICD in PD. Several neuroimaging studies of PD patients with ICD have revealed a maladaptive mesolimbic system and dysfunctional frontostriatal circuits, both of which are involved in conflict decision-making, impulse control, and monitoring of behaviors, with negative consequences.3,4 However, there is a paucity of data on the microstructural changes associated with ICD in PD. We hypothesized that both an underlying pathological process and a medication-related effect play significant roles in the development of ICD, since PD is a degenerative disorder. The aim of this study was to identify the brain microstructural changes in PD patients with and without ICD via whole-brain diffusion-tensor imaging (DTI).
The 42 right-handed subjects that were prospectively enrolled in this study comprised 14 PD patients with ICD (PD-ICD), 10 PD patients without ICD (PD-nonICD), and 18 age-matched healthy controls (HCs). The diagnosis of PD was based on the United Kingdom Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria, and all subjects were followed up in the Movement Disorders Clinic at Seoul National University Hospital, Korea. The PD-ICD group included subjects exhibiting one or more behaviors that met the ICD criteria of the American Psychiatric Association (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, 1994) and who had no history of ICD before developing PD. The PD-nonICD group included subjects without ICD despite undergoing more than 5 years of dopamine-replacement therapy and who had no history of ICD before developing PD. A trained neuroradiologist examined T1-weighted and fluid-attenuated inversion recovery (FLAIR) magnetic resonance (MR) images to exclude subjects who exhibited any evidence of cortical infarction, leukoencephalopathy, hemorrhage, brain tumors, encephalitis, or severe white-matter lesions. Subjects with depression symptoms, cognitive impairment, psychiatric disorders other than ICD, a history of neurologic disorders other than PD, and a history of surgical treatment for PD were also excluded. Clinical information including age, gender, daily antiparkinsonian medication dosages, duration of PD, Unified Parkinson's Disease Rating Scale (UPDRS) score, Hoehn and Yahr (HY) stage, Geriatric Depression score, and Mini-Mental State Examination score were collected for all subjects. The Institutional Review Board of Seoul National University Hospital approved this study, and signed informed consents to participate were obtained from all subjects.
Images were obtained for all subjects using a 3-T MR imaging (MRI) system (Trio, Siemens, Erlangen, Germany) with 30 noncollinear diffusion gradients and one zero gradient (B0 images). Images were acquired using a 32-channel head coil based on a spin-echo echo-planar image sequence with the following parameters: repetition time/echo time=10,000 ms/85 ms, b=1,000 s/mm2, matrix=128×128, FOV=240 mm, axial slice thickness=2.5 mm without gap, number of excitations=1, and 60 slices. All PD subjects underwent MRI scanning in a medication-on condition.
Raw diffusion images were masked using the Brain Extraction Tool from the Brain Analysis Toolbox in the FMRIB Software Library (FSL, FMRIB, University of Oxford, Oxford, UK). By using the masked and corrected diffusion image volumes as inputs, scalar maps of fractional anisotropy (FA) and mean diffusivity (MD) were extracted by applying the FMRIB DTIFIT tool. Images were preprocessed and nonlinearly registered onto FMRIB58_FA with the all-subjects-to-all-subjects option. The mean images were created according to MNI152 (Montreal Neurological Institute, McGill University, Quebec, Canada) standard space (1×1×1 mm3). The strong signals were denoted by skeletonizing the images with a fixed threshold of 0.2. Aligned images were projected onto the previously created skeleton image, thereby eliminating the effects of individual variant signals and misalignments. Each image was examined for possible artifacts caused during data acquisition before proceeding with further processing. All postprocessing analyses were performed using FSL Tract-Based Spatial Statistics.5
The qualities of the MR images and cortical surface were inspected by both a neurologist and an engineer. A trained engineer manually examined the images at every processing step. Images that did not meet the suitable qualities of T1-weighted MR images and cortical surfaces were excluded from the analyses. The main reasons for excluding patients were (a) signs of white-matter lesions visible on FLAIR MR images, (b) corrupted FA map of individual spaces, and (c) presence of any motion artifacts.
For the group comparisons of clinical characteristics, the Mann-Whitney U test was used for continuous variables and Fisher's exact test was used for categorical variables. Group comparisons of DTI data were performed on voxel-by-voxel basis using skeletonized images of the mean FA and MD. Group differences were analyzed in a general linear model representing the group-based dependence of FA or MD. Comparison between the HC and PD-nonICD or PD-ICD groups were controlled for the confounding factors of age and gender. Comparisons of PD-nonICD and PD-ICD images were controlled for the following confounding factors: age, gender, HY stage, PD duration, and equivalent daily dose of the dopamine agonist levodopa. The threshold probability value for the significance of group differences was corrected p<0.05. Statistical correction for multiple comparisons was performed using a threshold-free cluster-enhancement option, with height=2, extent=0.5, and neighbor connectivity=26. Voxel-wise statistics for scores were calculated by using the FSL nonparametric permutation test method (with 10,000 permutations).
Of the 42 initially enrolled subjects, 2 withdrew and the diffusion images for 3 were of markedly poor quality, hence data from 37 subjects (10 PD-ICD, 9 PD-nonICD, and 18 HCs) were included in the analyses. The demographic and clinical characteristics of these 37 subjects are summarized in Table 1. The PD duration, UPDRS score, HY stage, and daily dopaminergic medication dosage did not differ significantly between the two PD groups (Table 1). The details of the ICD behaviors of the 10 PD-ICD subjects are given in Supplementary Table 1 (in the online-only Data Supplement). Seven patients exhibited two or more types of ICD behaviors.
The FA in the bilateral orbitofrontal, medial prefrontal, and anterior cingulate areas was significantly lower in the PD-nonICD group than in the HC group (Fig. 1), whereas the FA in the anterior and dorsal corpus callosum was significantly higher in the PD-ICD group (Fig. 2). There were no regions with significantly low FA in the PD-ICD group. Furthermore, there were no significant differences in the MD maps between the HC and either of the two PD groups.
Fractional anisotropy was significantly higher in the PD-ICD group than in the PD-nonICD group for the anterior corpus callosum, partial left thalamic radiations, right dorsal and posterior cingula, right internal capsule (genu and posterior limbs), right superior temporo-occipital lobes, and right thalamic radiations (Fig. 3). There were no regions in which the FA was lower in the PD-ICD group than in PD-nonICD group, and no significant differences at all between the MD maps of these two groups.
The results of this study indicate that there are distinct changes in the diffusion tensors between PD patients with and without ICD. The whole-brain analysis performed in this study revealed widespread FA increases and no significant FA decreases in PD-ICD compared to PD-nonICD patients.
Several hypotheses have been proposed for explaining the high prevalence of ICD among PD patients. An association between reward-system deficiency and ICD has been suggested,6 since the mesolimbic dopaminergic system may also undergo degeneration in PD;7 however, a hyperdopamine theory related to dopaminergic medications in the relatively spared mesolimbic system is a more plausible mechanism.3,4 Hypoactivation of the orbitofrontal areas has been observed when pathological gamblers without PD are gambling, suggesting that reward-system deficiency is an underlying mechanism among pathological gamblers in the general population.8 Hypometabolism in the orbitofrontal cortex has also been reported in PD patients without ICD when performing Iowa gambling tasks.9 A study involving rats has also shown that the medial prefrontal cortex is particularly important in reward processing via dopaminergic input, since it forms part of the mesocorticolimbic dopaminergic pathways.10 The present study found that FA in the bilateral medial prefrontal and orbitofrontal cortex was lower in the PD-nonICD group than in the HC group, which suggests the presence of a reward-system deficiency in PD patients without ICD, since measures of FA indirectly represent the integrity of neuronal fibers. However, contrasting results were obtained for the PD-ICD patients.
The FA in the anterior corpus callosal areas, right internal capsule, right dorsal and posterior cingula, and right thalamic radiations was higher in the PD-ICD group than in the PD-nonICD group. These results suggest that the neural mechanism of ICD in PD differs from that in people with addictive disorders in the general population. The relative preservation of neural integrity in these areas might be a risk for medication-related ICD in the PD population. Comparison of the PD-ICD and PD-nonICD patients in the present study revealed that none of the regions in the dopaminergic pathways pertaining to reward processing exhibited decreased FA or increased MD, while tracts connecting the subthalamic structures and striatal regions exhibited higher FA. In line with this observation, cerebral blood flow in the right ventral pallidum and nucleus accumbens, as reflected by a single-photon-emission computed tomography scan, was reported to be higher in resting-state PD patients exhibiting pathological gambling.3 This combined evidence of high activation levels in regions related to reward processing demonstrates that PD patients with ICD may have more intact reward-processing circuits and better preserved dopaminergic pathways than their counterparts without ICD. Therefore, the present findings regarding structural differences between PD-ICD and PC-nonICD patients and HCs support the aforementioned hyperdopamine theory, suggesting that ICD is accentuated by high dosages of dopamine agonists that have high affinity to the reward-processing pathways, which in PD patients with ICD are not as damaged as in those without ICD.
The distinct DTI changes observed in our PD-ICD group may also have been caused by the establishment of ICD: frequent execution of uncontrolled behaviors in PD patients with ICD may result in a modification of tissue integrity, as reflected by the higher FA, which represent stronger directivity of neuronal fibers, contributing to greater neurotransmission between regions. The pathological tendency to repeat impulsive behaviors not only creates signal overflow but may also continuously remodel related structures and enhance neuronal communications.
Fractional anisotropy in the dorsal to anterior corpus callosum was significantly higher in the PD-ICD patients than in the HC subjects. The presence of prefrontal interconnections residing in the anterior corpus callosum has led to the suspicion that the degeneration in that region is functionally associated with the control of impulsivity and reward-craving behaviors such as addictions.11,12 In this context, increased FA in the corpus callosum may represent evidence of undamaged or strengthened prefrontal structures of ICD associated with reward functions. The characteristic compulsive nature of ICD in PD and the recorded neuronal overactivations are possible causes for these structural changes, as shown in patients with obsessive-compulsive disorder, who exhibit higher FA in the corpus callosum.13,14
This study represents a preliminary investigation of the whole-brain white-matter changes associated with medication-related ICD in PD; however, the results must be interpreted with caution due to several study limitations. The smallness of the sample renders it difficult to differentiate between group measures in specific regions. In addition, most of the PD subjects did not have severe motor complications or disabilities, despite prolonged disease, which could mean that some of the reported ICD-related changes were unrelated to PD progression. However, selection bias might also have been present due to the preferential inclusion of patients with relatively benign disease. In addition, the images were obtained in a medication-on state, although previous reports have indicated that dopaminergic drugs do not significantly alter FA in DTI.12 Patients exhibiting multiple ICD behaviors were included, and so the heterogeneity in behaviors may have impeded the harvesting of more specific results. Future studies should recruit more PD-ICD and nonICD patients in order to distinguish differences between HC subjects and these PD patients, and to draw more precise explanations on the highlighted regions. Furthermore, the insights into the structural changes in patients with and without ICD could be improved by using various methodologies including probabilistic tractography and multimodal imaging.
The pathophysiological mechanism underlying ICD in PD may be multifactorial and related to both the pathological progression of PD and the neural plastic changes associated with chronic dopaminergic therapy. Replication studies and further multimodal imaging studies are needed to reveal the complex mechanism underlying ICD in PD.
Figures and Tables
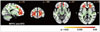 | Fig. 1Comparison of FA maps between PD-nonICD patients and HC subjects. PD-nonICD<HC, p<0.05 (corrected). FA: fractional anisotropy, HC: healthy control, ICD: impulse control disorder, MPFC: medial prefrontal cortex, PD: Parkinson's disease, SFC: superior frontal cortex. |
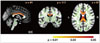 | Fig. 2Comparison of FA maps between PD-ICD patients and HC subjects. PD-ICD>HC, p<0.05 (corrected). CC: corpus callosum, FA: fractional anisotropy, HC: healthy control, ICD: impulse control disorder, PD: Parkinson's disease. |
 | Fig. 3Comparison of FA maps between PD-ICD patients and PD-nonICD patients. PD-ICD>PD-nonICD, p<0.05 (corrected). CC: corpus callosum, FA: fractional anisotropy, HC: healthy control, ICD: impulse control disorder, PD: Parkinson's disease, SN: substantia nigra. |
Table 1
Clinical characteristics of the subjects

Data shown as mean (SD) unless otherwise indicated.
*Comparison between CON and PD as a total group, †Comparison between PD-ICD and PD-nonICD groups.
CON: normal control, GDS: Geriatric Depression Scale, HY stage: Hoehn & Yahr stage, ICD: impulse control disorder, LED: levodopa equivalent dose, MMSE: Mini-Mental Status Examination, n/a: not available, PD: Parkinson's disease, PD-ICD: Parkinson's disease subjects with ICD, PD-nonICD: Parkinson's disease subjects without ICD, UPDRS: Unified PD Rating Scale.
Acknowledgements
This study was supported by a grant of National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0014451).
References
1. Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010; 67:589–595.
2. Lee JY, Kim JM, Kim JW, Cho J, Lee WY, Kim HJ, et al. Association between the dose of dopaminergic medication and the behavioral disturbances in Parkinson disease. Parkinsonism Relat Disord. 2010; 16:202–207.

3. Cilia R, Siri C, Marotta G, Isaias IU, De Gaspari D, Canesi M, et al. Functional abnormalities underlying pathological gambling in Parkinson disease. Arch Neurol. 2008; 65:1604–1611.

4. Joutsa J, Martikainen K, Niemelä S, Johansson J, Forsback S, Rinne JO, et al. Increased medial orbitofrontal [18F]fluorodopa uptake in Parkinsonian impulse control disorders. Mov Disord. 2012; 27:778–782.

5. Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006; 31:1487–1505.

6. Lawrence AD, Evans AH, Lees AJ. Compulsive use of dopamine replacement therapy in Parkinson's disease: reward systems gone awry? Lancet Neurol. 2003; 2:595–604.

7. Ouchi Y, Yoshikawa E, Okada H, Futatsubashi M, Sekine Y, Iyo M, et al. Alterations in binding site density of dopamine transporter in the striatum, orbitofrontal cortex, and amygdala in early Parkinson's disease: compartment analysis for beta-CFT binding with positron emission tomography. Ann Neurol. 1999; 45:601–610.

8. Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005; 8:147–148.

9. Thiel A, Hilker R, Kessler J, Habedank B, Herholz K, Heiss WD. Activation of basal ganglia loops in idiopathic Parkinson's disease: a PET study. J Neural Transm. 2003; 110:1289–1301.

10. Tzschentke TM. The medial prefrontal cortex as a part of the brain reward system. Amino Acids. 2000; 19:211–219.

11. Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005; 30:610–617.

12. Grant JE, Brewer JA, Potenza MN. The neurobiology of substance and behavioral addictions. CNS Spectr. 2006; 11:924–930.

Supplementary Material
The online-only Data Supplement is available with this article at http://dx.doi.org/10.3988/jcn.2015.11.1.42.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download