Abstract
Background and Purpose
The rate and outcomes of neurologic complications of patients receiving extracorporeal
membrane oxygenation (ECMO) are poorly understood. The purpose of this study was to
identify these parameters in ECMO patients.
Methods
All patients receiving ECMO were selected from the Nationwide Inpatient Sample between
2001-2011. The rate and outcomes of neurologic complications [acute ischemic stroke,
intracranial hemorrhage (ICH), and seizures] among these patients was determined.
Discharge status, mortality, length of stay, and hospitalization costs were compared
between patients with and without neurologic complications using chi-squared tests for
categorical variables and Student's t-test for continuous
variables.
Results
In total, 23,951 patients were included in this study, of which 2,604 (10.9%)
suffered neurologic complications of seizure (4.1%), stroke (4.1%), or ICH
(3.6%). When compared to patients without neurologic complications, acute
ischemic stroke patients had significantly higher rates of discharge to a long-term
facility (12.2% vs. 6.8%, p<0.0001) and a
significantly longer mean length of stay (41.6 days vs. 31.9 days,
p<0.0001). ICH patients had significantly higher rates of
discharge to a long-term facility (9.5% vs. 6.8%,
p=0.007), significantly higher mortality rates (59.7% vs.
50.0%, p<0.0001), and a significantly longer mean length
of stay (41.8 days vs. 31.9 days) compared to patients without neurologic complications.
These outcomes did not differ significantly between seizure patients and patients
without neurologic complications.
Extracorporeal membrane oxygenation (ECMO) is an increasingly used technique providing
cardiopulmonary support to patients with severe refractory cardiac and respiratory failure.
Standard ECMO involves venous drainage from the femoral vein or left atrium with artificial
extracirculatory oxygen exchange and return of the blood to the body through the same veins
(venovenous) or to the arterial system through the femoral artery or ascending aorta
(venoarterial).1 ECMO is commonly used in neonates
and infants with congenital heart defects or respiratory distress syndrome, and it is also
increasingly being used as a transplant bridge in adults who survive cardiopulmonary
resuscitation, and in patients with cardiogenic shock and severe respiratory failure.23
Some studies have demonstrated that neurologic complications are rather common among
patients receiving ECMO.4 These complications are
generally related to thrombosis with infarction, or hemorrhage.5 Intracranial hemorrhage (ICH) in particular has been associated with
higher rates of mortality.46 However, much of the evidence regarding the incidence and outcomes of
neurologic complications among ECMO patients is limited to small case series.7
A large, multihospital database, the Nationwide Inpatient Sample (NIS) of the Healthcare
Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality, was
studied between 2001-2011. We sought to characterize the rate and outcomes of neurologic
complications among patients receiving ECMO.
The NIS hospital discharge database for the period 2001-2011 was obtained from the HCUP
of the Agency for Healthcare Research and Quality. This database represents 20% of
all inpatient discharges from nonfederal hospitals in the United States. Patients included
in this study were selected using the International Classification of Diseases 9th
Revision (ICD-9) procedure code for extracorporeal membranous oxygenation, 3965.
The demographic information analyzed included age and gender. Age was categorized into
neonates (<1 month), infants (1 month to 1 year), children (1-17 years), adults
(18-64 years), and seniors (≥65 years). These age groups were similar to those in
the Extracorporeal Life Support Organization (ELSO) registry, with the exception that
adults were defined as aged ≥18 years in the present study and a separate group of
patients aged ≥65 years was also included.8
Comorbidities and complications included in this study were congenital heart disease
[clinical classification software code (CCS): 213], sepsis (CCS: 2), cardiac arrest (CCS:
107), shock (CCS: 249), respiratory distress syndrome (CCS: 221), and respiratory failure,
insufficiency, arrest (CCS: 131). The prevalence of these comorbidities among ECMO
patients in each age group was studied. The Charlson Comorbidity Index was calculated for
each patient.9
The primary endpoints of this study were the following neurologic complications: ICH
(ICD-9: 430, 431, 4320, 4321, 4329), acute ischemic stroke (ICD-9: 433.X1, 434.X1, 436,
437.0, 437.1, 437.4, 437.5, 437.7, 437.8, and 437.9), and seizure (CCS: 83). The discharge
status (discharge to home or discharge to long-term facility), in-hospital mortality,
length of stay, and 2011-adjusted hospitalization costs of patients with neurologic
complications with patients who did not suffer neurologic complications were compared.
Rates of neurologic complications were also compared across age groups and
comorbidities/indications. A separate analysis of the rate of neurologic complications was
also performed by age group, and trends in the utilization of ECMO during the period
2001-2011 were examined.
Chi-squared tests were used to compare categorical variables, and the Student's
t-test was used to compare continuous variables, assuming a cutoff for
statistical significance of p<0.05. Probability values for
outcomes were obtained using the no-neurologic-complications group as a reference group.
Discharge weights were applied to make the data nationally representative. Two
multivariate analyses were performed; one was designed to determine the comorbidities and
demographic characteristics associated with neurologic complications (stroke, ICH, or
seizure) and in-hospital mortality; all comorbidities, indications, and demographic
factors were forced into this model. Except where stated otherwise, the data are presented
as mean±SD values. All statistical analyses were performed using the SAS-based
statistical package JMP (www.jmp.com).
In total, 23,951 patients were included in this study. The number of patients receiving
ECMO increased from 1,613 patients in 2001 to 3597 patients in 2011 (Fig. 1). The number of pediatric patients receiving ECMO increased from
1,027 to 1,434 during the same time period, while the number of adult ECMO patients
increased from 309 to 2004 (Fig. 1). Age data were
available for 21,304 patients; these patients were 18.5±56.3 years old, and 7,741
patients (36.3%) were younger than 1 month and 8,398 patients (39.4%) were
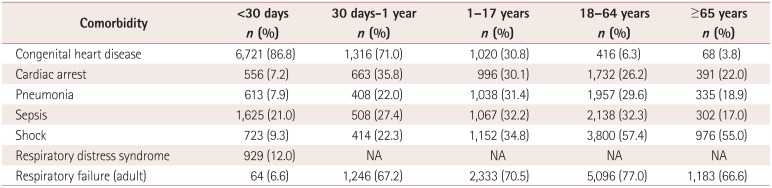
≥18 years old. The most common indication/comorbidity was congenital heart disease
(n=11,712, 48.9%) followed by adult respiratory failure
(n=11,407, 47.6%) and shock (n=7,369,
30.8%). These data are summarized in Table
1. The most common indications/comorbidities by age group were congenital heart
disease for patients aged <1 month (86.8%) and aged between 1 month and 1
year (71.0%). Respiratory failure was the most common indication in patients aged
1-17 years (70.5%), 18-64 years (77.0%), and ≥65 years
(66.6%). These data are summarized in Supplementary Table 1 (in the online-only Data Supplement).
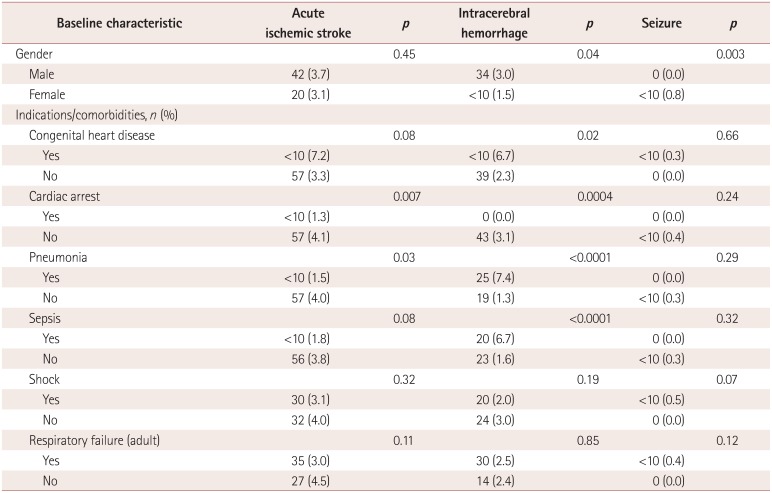
A total of 2,604 patients (10.9%) suffered neurologic complications of seizure
(n=986, 4.1%), stroke (n=980, 4.1%), or
ICH (n=862, 3.6%). When compared to patients without neurologic
complications, acute ischemic stroke patients had a significantly higher rate of discharge
to a long-term facility (12.2% vs. 6.8%, p<0.0001),
a significantly longer mean length of stay (41.6 days vs. 31.9 days,
p<0.0001), and significantly higher costs (US$ 639,528 vs.
US$ 501,184, p<0.0001). When compared to patients without
neurologic complications, ICH patients had a significantly higher rate of discharge to a
long-term facility (9.5% vs. 6.8%, p=0.007), a
significantly higher mortality rate (59.7% vs. 50.0%,
p<0.0001), a significantly longer mean length of stay (41.8 days
vs. 31.9 days, p=0.01), and significantly higher hospitalization costs
(US$ 641,636 vs. US$ 501,184, p=0.002). The outcomes did
not differ significantly between seizure patients and patients without neurologic
complications. These data are summarized in Table
2.
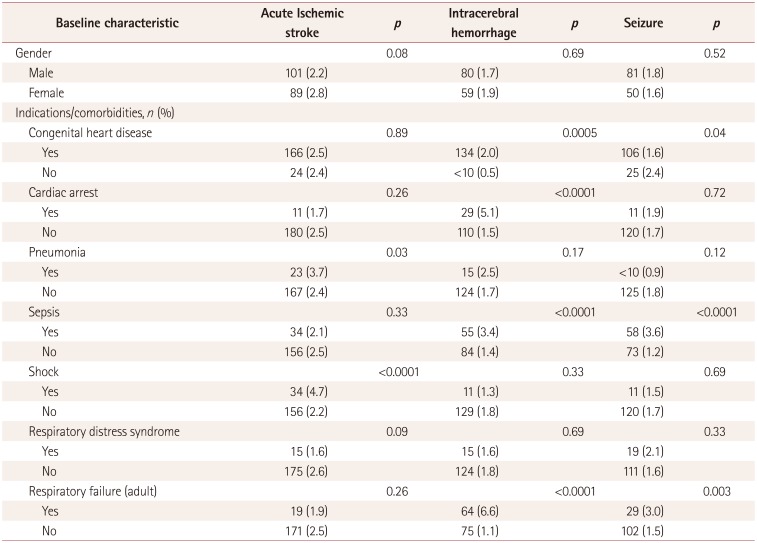
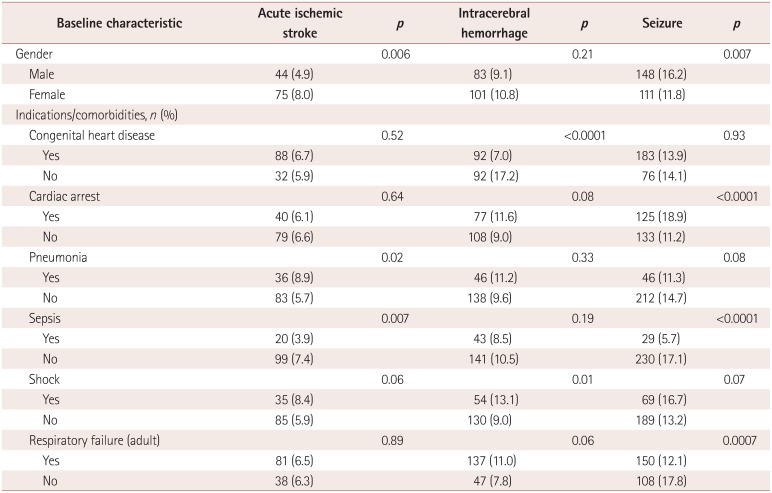
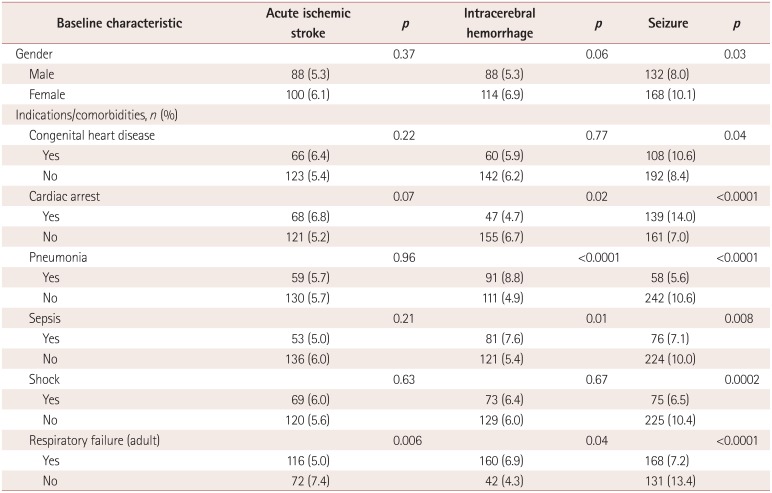
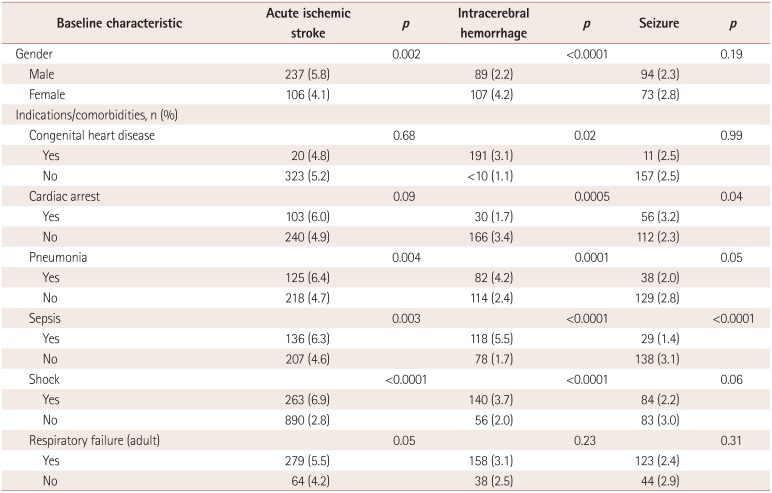
The results of univariate analyses of variables associated with neurologic complications
are presented in Table 3. Patients aged <1
month had the lowest rate of acute ischemic stroke (2.5%), while patients aged
between 1 month and 1 year had the highest rate (6.4%,
p<0.0001). Patients with shock had the highest rate of acute
ischemic stroke (6.0% vs. 3.2%, p<0.0001), while
those with respiratory distress syndrome had the lowest rate (2.5% vs. 4.2%,
p<0.0001). Patients aged <1 month also had the lowest
rate of ICH (1.8%), while those aged between 1 month and 1 year had the highest
rate (9.9%, p<0.0001). Respiratory distress syndrome was
associated with the lowest rate of ICH (1.2% vs. 3.7%,
p<0.0001). Patients aged <1 month had the lowest rate of
seizure (1.7%), while those aged between 1 month and 1 year had the highest
(13.9%, p<0.0001). Cardiac arrest was the comorbidity
associated with the highest rate of seizure (8.3% vs. 3.1%,
p<0.0001), and respiratory distress syndrome was associated with
the lowest rate (2.0% vs. 4.2%, p<0.0001). The
results of analyses of variables associated with neurologic complications by age are
summarized in Supplemental Table
2, 3, 4, 5, 6 (in the online-only Data
Supplement).
Variables predictive of neurologic complications (stroke, ICH, or seizure) on
multivariate analysis included cardiac arrest [odds ratio (OR)=1.30, 95% confidence
interval (CI)=1.17-1.45, p<0.0001], sepsis (OR=1.12,
95%CI=1.01-1.24, p=0.03), and shock (OR=1.24,
95%CI=1.12-1.37, p<0.0001). Compared to neonates, all age
groups had significantly lower odds of neurologic complications on multivariate analysis,
with the risk being highest for infants aged between 1 month and 1 year. These data are
summarized in Table 4.
Variables predictive of mortality on multivariate analysis included cardiac arrest
(OR=1.49, 95%CI=1.38-1.60, p<0.0001), sepsis (OR=1.12,
95%CI=1.05-1.20, p=0.0009), shock (OR=1.20,
95%CI=1.13-1.29, p<0.0001), and respiratory failure
(OR=1.16, 95%CI=1.08-1.25, p<0.0001). ICH was the only
neurologic complication associated with higher odds of mortality (OR=2.31,
95%CI=1.89-2.84, p<0.0001). Adult patients (aged 18-64
years) had significantly higher odds of mortality compared with the pediatric cohort
(OR=1.88, 95%CI=1.69-2.09, p<0.0001), as did seniors (aged
≥65 years; OR=2.34, 95%CI=2.04-2.68, p<0.0001).
These data are summarized in Table 4.
The findings of this study show that the rates of ICH, acute ischemic stroke, and seizure
among patients receiving ECMO were each approximately 4%, but nearly 11% of
patients treated with ECMO had one of these neurologic complications. Compared to patients
without neurologic complications, those suffering neurologic complications of acute ischemic
stroke and ICH had higher rates of discharge to a long-term facility, and ICH patients had
higher rates of mortality. Lengths of stay and hospitalization costs were also significantly
higher among acute ischemic stroke and ICH patients. It was also found that the rate of
neurologic complications was lower among neonates than in the other age groups. Furthermore,
patients with cardiac arrest and shock had significantly higher rates of neurologic
morbidity and mortality than those without these conditions.
The present findings also demonstrated that the number of patients receiving ECMO in the
United States is increasing rapidly, especially among adults. There was a 40%
increase in the number of pediatric patients receiving ECMO during the period 2001-2011, and
an impressive 650% increase in the number of adult patients receiving ECMO. Given
these rapid increases in the number of patients receiving ECMO, it is important to have a
comprehensive understanding of the incidence of neurologic complications among them. Several
studies have shown that patients suffering neurologic complications while on ECMO have
higher rates of long-term disability and suffer significantly higher morbidity and mortality
rates.1011
The present study confirms this finding, albeit on a much larger scale, and also shows that
hospitalization costs are more than US$ 100,000 higher among patients with neurologic
complications than among those without such complications. Given the higher rates of
morbidity and mortality and the high costs associated with neurologic complications, future
studies should investigate the development of methods for preventing neurologic
complications such as ischemic stroke and ICH.
Several studies have highlighted the high rates of mortality among adult ECMO
patients.121314 In a series of 87 adult patients,
Matteen et al.4 found a stroke rate of 8%, an
ICH rate of 10%, and a seizure rate of 0%. Overall, 50% of patients in
their series suffered neurologic complications defined as stroke, ICH, seizure,
encephalopathy, brain death, or coma. Similar to the present study, Matteen et al.4 found that increasing age was associated with higher
rates of death and neurologic morbidity. In a series of 607 adult patients, Lan et al.10 found that ischemic and hemorrhagic stroke affected
7% of the patients and was associated with significantly higher odds of death. In a
meta-analysis of 1,866 adult patients with cardiogenic shock, Cheng et al.15 found that stroke occurred in approximately 6%
of patients, which is a similar rate to rate of approximately 5% reported herein.
The neurologic outcomes of pediatric patients receiving ECMO have been studied
previously,16 and several risk factors for central
nervous system complications have been identified, including the use of
vasopressor/inotropic medications, infection, serum bicarbonate, pulmonary failure,
acidosis, elevated creatinine, and myocardial stunning.17 In a retrospective review of approximately 5,000 pediatric patients (aged 1
month to 18 years) from the ELSO database receiving ECMO, Cengiz et al.17 found that approximately 13% of patients developed acute severe
central nervous system complications, with the rate of neurologic complications and
mortality increasing with age. Other studies from the ELSO registry have found rates of
ischemic stroke of 7.4% and 4% in neonates and children, respectively, and ICH
rates of 7% and 6%. Clinical seizures were reported in 9.4% of neonates
and 5.9% of children.18 Similar rates of
neurologic complications are reported herein; however, neonates had significantly lower
rates of neurologic complications than other pediatric patients in the present study.
This study was subject to certain limitations. First, it was not possible to distinguish
between patients receiving venoarterial and venovenous ECMO. It is possible that
complication rates differ according to the type of ECMO received by patients. However,
many studies comparing venoarterial to venovenous ECMO have found no difference in
complication rates.192021 Second, it was also not
possible to determine whether the duration of ECMO support was associated with neurologic
complications, since this information was not available in the NIS database. Neurologic
complications were instead identified using discharge data in the form of ICD-9 codes;
therefore, the data are potentially subject to coding errors. Third, long-term outcomes
among patients included in this database could not be studied, and it was not possible to
determine whether any particular neurologic complication seen in this study resulted from
the original insult that triggered the use of ECMO or if it was a complication of the ECMO
procedure itself. Whether ECMO is a risk factor for neurologic complications per se, or if
patients with these specific neurologic conditions are just more likely to require ECMO
has yet to be ascertained. Fourth, it is possible that many of these patients were not
examined with neuroimaging; thus, the incidence of stroke or ICH could be underestimated.
One neuroimaging and neuropathologic study of 74 children receiving ECMO found that more
than 20% of patients had ischemic infarctions,22 and another study using T2-weighted MRI among ECMO patients found that nearly
all imaging patients had microhemorrhages on MRI.11
Finally, no adjustment for multiple comparisons was made in the present analyses, and so
it is possible that some of the findings were due to chance. For example, multivariate
analysis of mortality predictors revealed that stroke and seizure patients had lower odds
of death than those who did not suffer these complications; this is almost certainly a
chance finding.
The utilization of ECMO is increasing exponentially in the United States, especially
among adult patients. Neurologic complications such as ICH, acute ischemic stroke, and
seizures affect approximately 11% of patients. ICH is an independent predictor of
mortality, while stroke is associated with a higher rate of discharge to a long-term
facility. Given the increasing utilization of ECMO and the high costs and poor outcomes
associated with neurologic complications, more research is needed to determine the best
way to prevent neurologic complications in this patient population.
References
1. Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically
ill adult patients. Heart Lung Circ. 2008; 17(Suppl 4):S41–S47. PMID: 18964254.
2. Bulas DI, Taylor GA, O'Donnell RM, Short BL, Fitz CR, Vezina G. Intracranial abnormalities in infants treated with extracorporeal membrane
oxygenation: update on sonographic and CT findings. AJNR Am J Neuroradiol. 1996; 17:287–294. PMID: 8938301.
3. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza
Investigators. Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute
Respiratory Distress Syndrome. JAMA. 2009; 302:1888–1895. PMID: 19822628.
4. Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE, Schears GJ, Wijdicks EF. Neurological injury in adults treated with extracorporeal membrane
oxygenation. Arch Neurol. 2011; 68:1543–1549. PMID: 21825216.
5. Graziani LJ, Gringlas M, Baumgart S. Cerebrovascular complications and neurodevelopmental sequelae of neonatal
ECMO. Clin Perinatol. 1997; 24:655–675. PMID: 9394865.
6. Risnes I, Wagner K, Nome T, Sundet K, Jensen J, Hynås IA, et al. Cerebral outcome in adult patients treated with extracorporeal membrane
oxygenation. Ann Thorac Surg. 2006; 81:1401–1406. PMID: 16564280.
7. Cardarelli MG, Young AJ, Griffith B. Use of extracorporeal membrane oxygenation for adults in cardiac arrest
(E-CPR): a meta-analysis of observational studies. ASAIO J. 2009; 55:581–586. PMID: 19770800.
8. Paden ML, Conrad SA, Rycus PT, Thiagarajan RR. ELSO Registry. Extracorporeal Life Support Organization Registry Report
2012. ASAIO J. 2013; 59:202–210. PMID: 23644605.
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies:
development and validation. J Chronic Dis. 1987; 40:373–383. PMID: 3558716.
10. Lan C, Tsai PR, Chen YS, Ko WJ. Prognostic factors for adult patients receiving extracorporeal membrane
oxygenation as mechanical circulatory support--a 14-year experience at a medical
center. Artif Organs. 2010; 34:E59–E64. PMID: 20420591.
11. Liebeskind DS, Sanossian N, Sapo ML, Saver JL. Cerebral microbleeds after use of extracorporeal membrane oxygenation in
children. J Neuroimaging. 2013; 23:75–78. PMID: 22606942.
12. Fischer S, Bohn D, Rycus P, Pierre AF, de Perrot M, Waddell TK, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after
lung transplantation: analysis of the Extracorporeal Life Support Organization (ELSO)
registry. J Heart Lung Transplant. 2007; 26:472–477. PMID: 17449416.
13. Li J, Long C, Lou S, Hei F, Yu K, Wang S, et al. Venoarterial extracorporeal membrane oxygenation in adult patients:
predictors of mortality. Perfusion. 2009; 24:225–230. PMID: 19808747.
14. Massetti M, Tasle M, Le Page O, Deredec R, Babatasi G, Buklas D, et al. Back from irreversibility: extracorporeal life support for prolonged
cardiac arrest. Ann Thorac Surg. 2005; 79:178–183. discussion 183-184PMID: 15620939.
15. Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, et al. Complications of extracorporeal membrane oxygenation for treatment of
cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult
patients. Ann Thorac Surg. 2014; 97:610–616. PMID: 24210621.
16. Rehder KJ, Turner DA, Cheifetz IM. Extracorporeal membrane oxygenation for neonatal and pediatric respiratory
failure: an evidence-based review of the past decade (2002-2012). Pediatr Crit Care Med. 2013; 14:851–861. PMID: 24108118.
17. Cengiz P, Seidel K, Rycus PT, Brogan TV, Roberts JS. Central nervous system complications during pediatric extracorporeal life
support: incidence and risk factors. Crit Care Med. 2005; 33:2817–2824. PMID: 16352965.
18. Hervey-Jumper SL, Annich GM, Yancon AR, Garton HJ, Muraszko KM, Maher CO. Neurological complications of extracorporeal membrane oxygenation in
children. J Neurosurg Pediatr. 2011; 7:338–344. PMID: 21456903.
19. Kugelman A, Gangitano E, Pincros J, Tantivit P, Taschuk R, Durand M. Venovenous versus venoarterial extracorporeal membrane oxygenation in
congenital diaphragmatic hernia. J Pediatr Surg. 2003; 38:1131–1136. PMID: 12891480.
20. Rais-Bahrami K, Van Meurs KP. Venoarterial versus venovenous ECMO for neonatal respiratory
failure. Semin Perinatol. 2014; 38:71–77. PMID: 24580762.
21. Zahraa JN, Moler FW, Annich GM, Maxvold NJ, Bartlett RH, Custer JR. Venovenous versus venoarterial extracorporeal life support for pediatric
respiratory failure: are there differences in survival and acute
complications? Crit Care Med. 2000; 28:521–525. PMID: 10708194.
22. Lazar EL, Abramson SJ, Weinstein S, Stolar CJ. Neuroimaging of brain injury in neonates treated with extracorporeal
membrane oxygenation: lessons learned from serial examinations. J Pediatr Surg. 1994; 29:186–190. discussion 190-191PMID: 8176589.
Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.3988/jcn.2015.11.4.383.
jcn-11-383-s001.pdf
Supplementary Table 1
Prevalence of risk factors by age group

jcn-11-383-s002.pdf
Supplementary Table 2
Risk factors and neurological complications for patients <30 days old

jcn-11-383-s003.pdf
Supplementary Table 3
Risk factors and neurological complications for patients 30 days old to 1 year old

jcn-11-383-s004.pdf
Supplementary Table 4
Risk factors and neurological complications for 1 year-17 years old

jcn-11-383-s005.pdf
Supplementary Table 5
Risk factors and neurological complications for 18-64 years old

jcn-11-383-s006.pdf
Supplementary Table 6
Risk factors and neurological complications for 65 years old and older

Fig. 1
Number of patients treated with extracorporeal membrane oxygenation (ECMO) during the period 2001-2011. Graphical representation of ECMO utilization in the United States Nationwide Inpatient Sample from the period 2001-2011. ECMO utilization increased from 1,613 patients in 2001 to 3,597 patients in 2011. Utilization in adults increased from 309 patients in 2001 to 2004 patients in 2011; these data for the pediatric population are 1,027 and 1,434 patients, respectively.
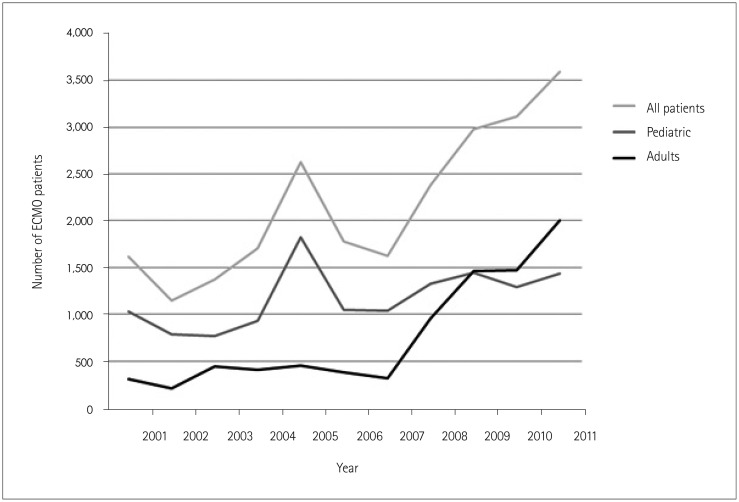
Table 1
Demographics and outcomes
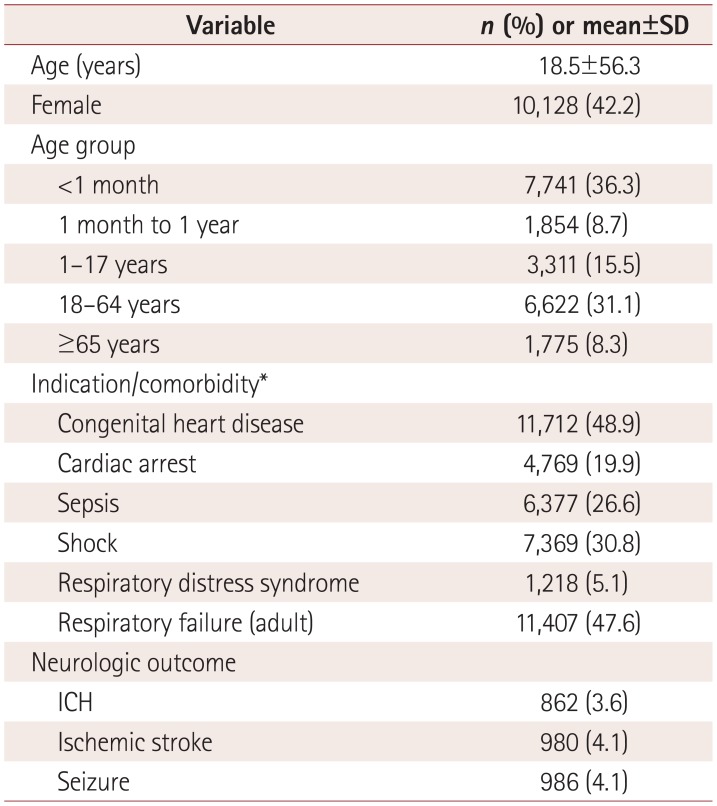
Table 2
Outcomes of ECMO-associated neurologic complications
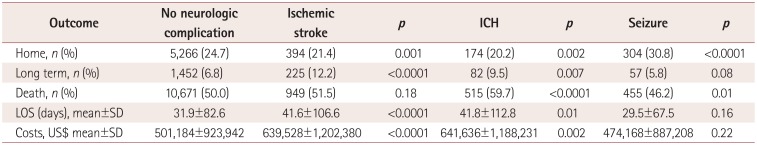
Table 3
Rate of neurologic complications by demographic, comorbidity, and indication characteristics
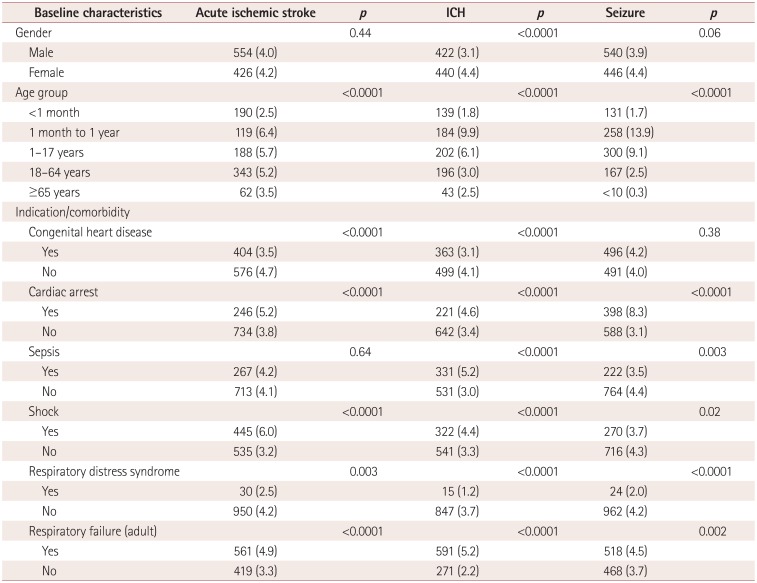
Table 4
Multivariate predictors of mortality




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download