1. Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007; 68:1583–1587.

2. König IR, Ziegler A, Bluhmki E, Hacke W, Bath PM, Sacco RL, et al. Predicting long-term outcome after acute ischemic stroke: a simple index works in patients from controlled clinical trials. Stroke. 2008; 39:1821–1826.
3. Sumer MM, Ozdemir I, Tascilar N. Predictors of outcome after acute ischemic stroke. Acta Neurol Scand. 2003; 107:276–280.

4. Nedeltchev K, der Maur TA, Georgiadis D, Arnold M, Caso V, Mattle HP, et al. Ischaemic stroke in young adults: predictors of outcome and recurrence. J Neurol Neurosurg Psychiatry. 2005; 76:191–195.

5. Kim JS. Pure lateral medullary infarction: clinical-radiological correlation of 130 acute, consecutive patients. Brain. 2003; 126(Pt 8):1864–1872.

6. Kim JS, Choi-Kwon S. Sensory sequelae of medullary infarction: differences between lateral and medial medullary syndrome. Stroke. 1999; 30:2697–2703.
7. Norrving B, Cronqvist S. Lateral medullary infarction: prognosis in an unselected series. Neurology. 1991; 41(2(Pt 1)):244–248.
8. Caplan LR. Bilateral distal vertebral artery occlusion. Neurology. 1983; 33:552–558.

9. Nelles G, Contois KA, Valente SL, Higgins JL, Jacobs DH, Kaplan JD, et al. Recovery following lateral medullary infarction. Neurology. 1998; 50:1418–1422.

10. Aydogdu I, Ertekin C, Tarlaci S, Turman B, Kiylioglu N, Secil Y. Dysphagia in lateral medullary infarction (Wallenberg's syndrome): an acute disconnection syndrome in premotor neurons related to swallowing activity? Stroke. 2001; 32:2081–2087.

11. Caplan LR, Pessin MS, Scott RM, Yarnell P. Poor outcome after lateral medullary infarcts. Neurology. 1986; 36:1510–1513.

12. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003; 42:1206–1252.

13. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497.
14. Vijan S. Type 2 diabetes. Ann Intern Med. 2010; 152:ITC31–ITC15. quiz ITC316

15. Martino R, Pron G, Diamant N. Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000; 15:19–30.

16. Perry L, Love CP. Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001; 16:7–18.

17. Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999; 30:1538–1541.

18. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006; 332:1077–1079.

19. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.

20. Hosoya T, Nagahata M, Yamaguchi K. Prevalence of vertebral artery dissection in Wallenberg syndrome: neuroradiological analysis of 93 patients in the Tohoku District, Japan. Radiat Med. 1996; 14:241–246.
21. Hosoya T, Watanabe N, Yamaguchi K, Kubota H, Onodera Y. Intracranial vertebral artery dissection in Wallenberg syndrome. AJNR Am J Neuroradiol. 1994; 15:1161–1165.
22. Shin JH, Suh DC, Choi CG, Leei HK. Vertebral artery dissection: spectrum of imaging findings with emphasis on angiography and correlation with clinical presentation. Radiographics. 2000; 20:1687–1696.

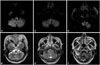
23. Kim JS, Lee JH, Suh DC, Lee MC. Spectrum of lateral medullary syndrome. Correlation between clinical findings and magnetic resonance imaging in 33 subjects. Stroke. 1994; 25:1405–1410.

24. Leno C, Berciano J, Combarros O, Polo JM, Pascual J, Quintana F, et al. A prospective study of stroke in young adults in Cantabria, Spain. Stroke. 1993; 24:792–795.

25. Leys D, Bandu L, Hénon H, Lucas C, Mounier-Vehier F, Rondepierre P, et al. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology. 2002; 59:26–33.

26. Dash D, Bhashin A, Pandit AK, Tripathi M, Bhatia R, Prasad K, et al. Risk factors and etiologies of ischemic strokes in young patients: a tertiary hospital study in north India. J Stroke. 2014; 16:173–177.

27. Kim H, Chung CS, Lee KH, Robbins J. Aspiration subsequent to a pure medullary infarction: lesion sites, clinical variables, and outcome. Arch Neurol. 2000; 57:478–483.

28. Kwon M, Lee JH, Kim JS. Dysphagia in unilateral medullary infarction: lateral vs medial lesions. Neurology. 2005; 65:714–718.

29. Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. J Neurol Neurosurg Psychiatry. 1989; 52:236–241.

30. Smithard DG, O'Neill PA, Parks C, Morris J. Complications and outcome after acute stroke. Does dysphagia matter? Stroke. 1996; 27:1200–1204.
31. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005; 36:2756–2763.
32. Bours GJ, Speyer R, Lemmens J, Limburg M, de Wit R. Bedside screening tests vs. videofluoroscopy or fibreoptic endoscopic evaluation of swallowing to detect dysphagia in patients with neurological disorders: systematic review. J Adv Nurs. 2009; 65:477–493.

33. Dávalos A, Ricart W, Gonzalez-Huix F, Soler S, Marrugat J, Molins A, et al. Effect of malnutrition after acute stroke on clinical outcome. Stroke. 1996; 27:1028–1032.

34. Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, et al. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003; 23:845–854.

35. Petcu EB, Sfredel V, Platt D, Herndon JG, Kessler C, Popa-Wagner A. Cellular and molecular events underlying the dysregulated response of the aged brain to stroke: a mini-review. Gerontology. 2008; 54:6–17.








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download