Abstract
Case Report
A 46-year-old male presented with progressive dizziness and imbalance of 3 weeks duration. The patient exhibited catch-up saccades during bedside horizontal HIT to either side, which was more evident during the rightward HIT. However, results of bithermal caloric tests and rotatory chair test were normal. MRI revealed a lesion in the inferior cerebellum near the flocculus.
Conclusions
This case provides additional evidence that damage to the flocculus or its connections may impair the vestibulo-ocular reflex only during high-speed stimuli, especially when the stimuli are applied to the contralesional side. By observing accompanying cerebellar signs, the abnormal HIT findings caused by a cerebellar disorder can be distinguished from those produced by peripheral vestibular disorders.
The head impulse test (HIT) can be used to evaluate the vestibulo-ocular reflex (VOR) during high-speed stimuli, and is an effective bedside tool for differentiating between central and peripheral vestibular disorders.12 The presence of corrective catch-up saccades during the HIT usually indicates the presence of a peripheral vestibular lesion.1 However, a false-positive HIT in patients with cerebellar ataxia with caloric responsiveness has been reported.3 Although exact neuroanatomical correlates remain unknown, a recent study has indicated the flocculus to be a potential candidate.4 We report abnormal HIT findings in another patient with a unilateral circumscribed cerebellar lesion near the flocculus.
A 46-year-old male presented with progressive spontaneous vertigo and disequilibrium of 3 weeks duration. A clinical examination revealed left-beating spontaneous nystagmus only (without fixation), augmented spontaneous nystagmus after horizontal head-shaking, direction-changing horizontal gaze-evoked nystagmus (GEN) that was more prominent during leftward gaze, impaired horizontal and vertical smooth pursuit that were more pronounced upward and to the left (Supplementary Video 1 in the online-only Data Supplement), and hypometric saccades in both the horizontal and vertical directions. The findings of a bedside HIT were abnormal in both horizontal directions, revealing decreased gain, and this was more evident during rightward head rotation (Supplementary Video 2 in the online-only Data Supplement). The direction of spontaneous nystagmus was not changed during positional maneuvers, and head-tilt and skew-deviation were absent. The patient fell to the left on the Romberg test, and dysmetria was evident to the left in finger-to-nose and heel-to-shin tests.
Pure-tone audiometry and bithermal caloric tests (12% of right-side canal paresis and 2% of left-side directional preponderance) produced normal results. The patient exhibited normal gains (from 0.24 at 0.01 Hz to 0.68 at 0.64 Hz) and phases of the VOR without asymmetry during sinusoidal harmonic accelerations (0.01-0.64 Hz, peak angular velocity=60°/s). The patient also exhibited normal time constants (rightward=9 s, leftward=11 s; normal range=from 5 to 25 s) during step-velocity rotation (initial angular acceleration of 100°/s2 for 1 s, followed by constant velocity rotation of 100°/s). The visually enhanced VOR was normal (0.83 at 0.08 Hz, normal range >0.8), but cancellation of the VOR (0.45 at 0.08 Hz, normal range <0.2) was impaired.
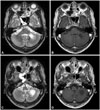
Serology revealed positive human immunodeficiency virus antigen and antibody, which was subsequently confirmed by Western blotting. The serum venereal disease research laboratory titer was low (1:2), and the fluorescent treponemal antibody absorption test was negative. Evaluations of autoimmune (antinuclear, antineutrophil cytoplasmic, and anti-Ro, -La, and -Jo-1 antibodies) and paraneoplastic conditions were unremarkable. CD4+ T cells were decreased to 137/mm3 (8.1%; normal range=27-60%). The CSF profile was normal, with negative viral (antibodies for herpes simplex types 1 and 2, varicella zoster, Epstein-Barr, cytomegalovirus, enterovirus, and John Cunningham virus), bacterial, fungal (cryptococcus and aspergillosis), tuberculosis, and parasitic markers. MRI revealed a round lesion located in the biventor lobule adjacent to the flocculus, tonsil, and inferior cerebellar peduncle (Fig. 1). Although antiretroviral agents were initiated with a presumptive diagnosis of progressive multifocal leukoencephalopathy associated with acquired immunodeficiency syndrome, the patient's symptoms were not relieved.
Our patient exhibited a decreased gain of the VOR only during high-speed angular stimuli, as documented by bedside HIT. In contrast, the VOR during low-speed and low-frequency stimuli, such as bithermal caloric testing and rotatory chair testing, remained intact. The lesion was adjacent to the flocculus, paraflocculus (tonsil), deep cerebellar nuclei, and inferior and middle cerebellar peduncles. Although the ipsilesional limb and truncal ataxia might have been caused by disrupted connections from the dentate and interposed nuclei, the absence of ocular lateropulsion and hypermetric saccades to either side of the lesion could have reflected a relative sparing of outputs from the oculomotor region of the fastigial nucleus.5 The connections between the nodulus/uvula and vestibular nucleus seem to have been preserved, given that positional nystagmus and the ocular tilt reaction were absent. The preferential involvement of the VOR only during high-speed stimuli has recently been described in a patient with an isolated floccular infarction.4 Moreover, the presence of GEN, decreased smooth pursuit, and impaired visual cancellation of the VOR suggest that disrupted connections from the flocculus and paraflocculus were responsible for the ocular motor findings in our patient.
The flocculus/paraflocculus is known to modulate the VOR.5 Experimental removal of the flocculus/ventral paraflocculus was shown to impair the ability of the VOR to adapt to visual demands.678 The gain of the VOR after ablation of the bilateral flocculi has varied markedly among studies (from slightly enhanced to decreased to unchanged),789 and the role of the flocculus/paraflocculus in modulating the VOR gain remains to be determined. A dissociation of the VOR gains-increased during low-speed and low-frequency stimuli and decreased during high-speed and high-frequency stimuli-was recently reported in a patient with an isolated floccular infarction.4 The authors of that study postulated that the flocculus enhances the VOR gain during high-speed and high-frequency stimuli, such as head impulses. During low-speed and low-frequency angular head motion, the VOR is generated by both excitatory and inhibitory vestibular signals.5 Since the inhibitory vestibular signals are saturated around a head velocity at 180°/s due to the inhibitory cut-off,10 it is predicted that the VOR would be insufficient to compensate for the high head velocity. However, since the VOR can compensate for a head velocity of up to 400°/s with visual cues,11 a central mechanism needs to be implemented to enhance the gain of VOR during high-velocity stimuli.5 Therefore, our case report supports the hypothesis that the flocculus serves as the central facilitating mechanism of the VOR gain during the HIT. The mechanism by which a unilateral floccular lesion can decrease the VOR gain during the HIT to both sides remains unknown. However, the simple spike activities in floccular Purkinje cells exhibit both inphase and out-of-phase responses according to the head rotation.12 In addition, as suggested previously,4 the presence of floccular target neurons related to the VOR and a reciprocal inhibitory connection between the vestibular nuclei might explain the abnormal HIT in the presence of unilateral floccular lesions.
Our patient also exhibited impaired smooth pursuit and visual cancellation of the VOR. The flocculus and paraflocculus participate in the modulation of pursuit-related eye movements and visual-vestibular interactions.5 Descending pursuit signals from the medial superior temporal area project to the medial and superior vestibular nuclei via the flocculus/ventral paraflocculus and dorsal vermis.13 Neurons in the flocculus/ventral paraflocculus are known to be more engaged in ipsilateral pursuit. Purkinje cells in the flocculus that respond to the gaze velocity are also responsible for cancelling the VOR during passive eye-head tracking.14 Therefore, in our patient, the predominant impairment of leftward pursuit and the failure of VOR cancellation may be explained by disruption of the floccular afferents from the pontine nuclei or the efferent fibers to the vestibular nuclei. On the other hand, the predominant impairment of upward pursuit in our patient was an unexpected finding, since most of the Purkinje cells in the flocculus that respond to the vertical gaze velocity exhibit downward on-directions, which was linked to a possible mechanism of downbeat nystagmus.15 However, conversely, abundant downward pursuit connections may exert a protective role for downward pursuit if the lesion is not complete. Otherwise, the lesion in our patient might have predominantly involved the upward pursuit pathway. Indeed, microstimulation of the flocculus and ventral paraflocculus elicits ipsilateral and upward-pursuit eye movement in monkeys.16
The flocculus/paraflocculus receives inputs from the medial vestibular nucleus, the nucleus prepositus hypoglossi, and cell groups of the paramedian tract,171819 and serves as the neural integrator that compensates for the leaky brainstem neural integrator.20 Accordingly, floccular dysfunction may generate GEN that is more prominent during ipsiversive eccentric gaze, as seen in our patient.
A previous study demonstrated an abnormal HIT in a patient with a lesion selectively involving the flocculus and anterior part of the tonsil, whereas our patient had a lesion restricted to the biventer lobule, which indicates that floccular damage is not mandatory for abnormal HIT findings in cerebellar lesions.
Our case provides additional evidence that HIT findings can be abnormal in the presence of a unilateral cerebellar lesion, especially when the stimuli are applied to the contralesional side. In the presence of other central vestibular and oculomotor signs, as demonstrated in the present case, the corrective saccades during the HIT should not be considered as a sign of peripheral vestibular disorders, especially when they occur bilaterally.
Figures and Tables
Acknowledgements
Dr. J-S Kim serves as an Associate Editor of Frontiers in Neuro-otology and is on the editorial boards of the Journal of Korean Society of Clinical Neurophysiology, Research in Vestibular Science, Journal of Clinical Neurology, Frontiers in Neuro-ophthalmology, Journal of Neuro-ophthalmology, Journal of Vestibular Research, and Case Reports in Ophthalmological Medicine. He received research support from SK Chemicals, Co. Ltd. All other authors report no disclosures.
References
2. Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009; 40:3504–3510.

3. Kremmyda O, Kirchner H, Glasauer S, Brandt T, Jahn K, Strupp M. False-positive head-impulse test in cerebellar ataxia. Front Neurol. 2012; 3:162.

4. Park HK, Kim JS, Strupp M, Zee DS. Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol. 2013; 260:1576–1582.

5. Leigh RJ, Zee DS. The neurology of eye movements. 4th ed. New York: Oxford University Press;2006.
6. Lisberger SG, Miles FA, Zee DS. Signals used to compute errors in monkey vestibuloocular reflex: possible role of flocculus. J Neurophysiol. 1984; 52:1140–1153.

7. Robinson DA. Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol. 1976; 39:954–969.

8. Schultheis LW, Robinson DA. Directional plasticity of the vestibuloocular reflex in the cat. Ann N Y Acad Sci. 1981; 374:504–512.

9. Zee DS, Yamazaki A, Butler PH, Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981; 46:878–899.

10. Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971; 34:661–675.

11. Pulaski PD, Zee DS, Robinson DA. The behavior of the vestibulo-ocular reflex at high velocities of head rotation. Brain Res. 1981; 222:159–165.

12. Raymond JL, Lisberger SG. Neural learning rules for the vestibulo-ocular reflex. J Neurosci. 1998; 18:9112–9129.

13. Tabata H, Yamamoto K, Kawato M. Computational study on monkey VOR adaptation and smooth pursuit based on the parallel control-pathway theory. J Neurophysiol. 2002; 87:2176–2189.

14. Leung HC, Suh M, Kettner RE. Cerebellar flocculus and paraflocculus Purkinje cell activity during circular pursuit in monkey. J Neurophysiol. 2000; 83:13–30.

15. Glasauer S, Stephan T, Kalla R, Marti S, Straumann D. Up-down asymmetry of cerebellar activation during vertical pursuit eye movements. Cerebellum. 2009; 8:385–388.

16. Belknap DB, Noda H. Eye movements evoked by microstimulation in the flocculus of the alert macaque. Exp Brain Res. 1987; 67:352–362.

17. Büttner-Ennever JA, Horn AK. Pathways from cell groups of the paramedian tracts to the floccular region. Ann N Y Acad Sci. 1996; 781:532–540.

18. Cheron G, Escudero M, Godaux E. Discharge properties of brain stem neurons projecting to the flocculus in the alert cat. I. Medical vestibular nucleus. J Neurophysiol. 1996; 76:1759–1774.

Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.3988/jcn.2015.11.3.279.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download