Abstract
Background
The safety of repeated mechanical thrombectomy within the acute stroke period has not yet been clearly demonstrated. We describe herein a patient who was successfully treated with repeated mechanical thrombectomy within the acute index stroke period.
Case Report
A 50-year-old woman with atrial fibrillation presented with left-sided weakness caused by occlusion of the right middle cerebral artery (MCA). Emergent mechanical thrombectomy with the Solitaire device achieved complete recanalization. The left MCA occlusion redeveloped at 6 days after the first treatment, at which time her international normalized ratio (INR) was 2.3. Endovascular thrombectomy was reattempted rapidly and complete recanalization was achieved again. Her neurologic symptoms resolved after the thrombectomy.
Mechanical thrombectomy has been widely used to treat hyperacute ischemic stroke in recent years.1 This procedure can be useful even when chemical thrombolysis is not possible because of the potential risk of hemorrhagic complications.23 However, the safety of repeated mechanical thrombectomy within the acute index stroke period has not yet been clearly demonstrated. We report herein a patient who was successfully treated twice with mechanical thrombectomy due to recurrent cardioembolic stroke within the acute index stroke period. Light microscopy of the thromboemboli confirmed their cardioembolic origin.
A 50-year-old woman was admitted to our emergency room with left-sided weakness of 5 hours and 45 minutes duration. She had been taking warfarin due to chronic atrial fibrillation for 10 years (CHA2DS2 VASc score=2). An initial neurologic examination revealed dysarthria, left visual field defect, and face and limb weakness on the left side. Visuospatial neglect on the left side was also noted. Electrocardiography revealed an atrial fibrillation. Her international normalized ratio (INR) was 1.74. Brain computed tomography (CT) angiography revealed tandem occlusion of the right distal internal carotid artery (ICA) and right proximal middle cerebral artery (MCA) along with low densities in the right insula and the basal ganglia. Emergent mechanical thrombectomy was performed (Fig. 1A). A 0.58 mm microcatheter was navigated across the occlusion site to the M2 portion of the right MCA using a 0.36 mm microwire. After removing the microwire, a Solitaire (ev3 Inc., Irvine, CA, USA) 4/20 stent was introduced and placed spanning the entire length of the thrombus. The Solitaire stent was retrieved during compression of the right common carotid artery, 5 minutes after deployment. After four attempts using this procedure, recanalization of the right ICA and MCA was achieved with capturing of the occluding thrombus, but the M2 superior division remained occluded. Another attempt at mechanical thrombectomy of the right M2 was conducted and complete recanalization (thrombolysis in cerebral infarction score, 3) was achieved at 8 hours and 15 minutes after symptom onset. A delayed angiogram revealed complete recanalization without residual stenosis (Fig. 1B). Brain magnetic resonance imaging (MRI) performed on the next day revealed an acute infarction in the right insula and basal ganglia with patent flows in the right ICA and MCA (Fig. 1C). The patient had no neurologic sequelae on day 3 after stroke onset. She had been administered intravenous heparin, and switching to warfarin was attempted.
Transthoracic echocardiography performed 5 days after the stroke onset demonstrated the presence of a thrombus, severe spontaneous echo contrast in the left atrium with left-atrium enlargement (volume of 142.6 mL), and decreased left atrial appendage (LAA) emptying velocity (0.087 m/sec). On day 6 after symptom onset, a 22-mm-sized thrombus with marked left atrial enlargement was noted in the LAA on multichannel cardiac CT (Fig. 1D). Aphasia and right-sided hemiplegia developed 2 hours and 45 minutes after cardiac CT was performed. The patient's INR was 2.34. Brain CT angiography revealed a newly developed occlusion on the M1 segment of the left MCA. Endovascular thrombectomy was attempted again (Fig. 1E). Complete recanalization was achieved at 2 hours after symptom onset with the first attempt at suction thrombectomy and one trial mechanical thrombectomy with the Solitaire (ev3 Inc.) device for left M1 occlusion (Fig. 1F). The patient's neurologic symptoms again completely resolved immediately after successful thrombectomy. Diffusion-weighted MRI conducted the next day revealed a slightly high signal intensity in the left basal ganglia (Fig. 1G). Follow-up multichannel cardiac CT performed 3 days after the second event confirmed the disappearance of the previously observed thrombus in the left atrium, but a small residual thrombus was suspected in the LAA (Fig. 1H). The patient was discharged to home 10 days later.
Histologic studies were performed to investigate the nature of the thromboemboli that had been retrieved during the endovascular treatments for this patient. Hematoxylin and eosin (H&E) staining of the extracted thromboembolus of the index stroke revealed a large amount of fresh red blood cells (RBCs) and intact granulocytes (Fig. 2A and C), while the clot of the second stroke contained few intact RBCs and granulocyte apoptosis (Fig. 2D and F). However, antiglycophorin-A staining, which is used for highly selective detection of RBCs, revealed that both thrombi were mostly composed of RBCs (Fig. 2B and E).
Mechanical thrombectomy has several advantages over pharmacological thrombolysis, since it not only enables a longer treatment window but also achieves faster recanalization with a higher success rate,4 and it can be considered in cases of recent stroke or significant abnormalities in hemostasis or for patients with contraindications for chemical thrombolysis.3 However, the rates of symptomatic intracranial hemorrhage and procedure-related complications are reportedly 6.8-11.2% and 3.4-5.5%, respectively, in patients treated with mechanical thrombectomy.56 Previous studies have shown that neurologic complications can increase when patients with previous stroke are treated with an endovascular procedure.7 The case described herein received mechanical thrombectomy for the index stroke because of more than 4.5 hours after symptom onset and a relatively high INR, which is a contraindication for intravenous thrombolysis. In the second stroke event, mechanical thrombectomy was reconsidered primarily because of the higher bleeding risk with thrombolytic agents due to the sustained high INR and the symptomatic ischemic lesion that had appeared 6 days previously. This patient ultimately fully recovered without any procedure-related complication. Thus, repeated mechanical thrombectomy can be safely and successfully performed in patients with a high INR and a recurrent stroke within the acute index stroke period.
We considered that the mechanism of both stroke events in the present case to be cardioembolism. Our patient had atrial fibrillation, which is known to be most common cause of cardioembolism, and there was no culprit lesion in the cerebral artery after successful recanalization. We confirmed that the thrombus originated from the heart in the second stroke event, since the thrombus detected on multichannel cardiac CT had disappeared in the follow-up cardiac CT.
Our patient unexpectedly developed a recurrent cardioembolic stroke during hospitalization, despite her sustained INR of 2.34. The reported frequency of early cardioembolic stroke recurrence has varied between 1% and 22%.8 The risk factors for early embolic recurrence in cardioembolic infarction are alcohol abuse, hypertension with valvular heart disease and atrial fibrillation, nausea and vomiting, and previous cerebral infarct.8 Although the therapeutic intensity of warfarin therapy is effective at preventing embolic events, previous reports have suggested that a history of previous thromboembolic events and LAA abnormalities are predictive factors for the development of cerebral embolism.9 Our patient had a history of cardioembolic stroke 1 week before and severe spontaneous echo contrast in the LAA with a decreased LAA emptying velocity. These features, which are suggestive of pronounced cardiac abnormality in atrial fibrillation, may be related to sustained thrombogenesis despite appropriate anticoagulation.
The mechanism underlying the production of a red thrombus differs from that of a platelet-rich thrombus (white thrombus). Atrial fibrillation promotes abnormal blood flow with turbulence and blood stasis, which enhances procoagulant activity by endothelial activation. This disrupts the laminar flow that is necessary to prevent the wash out and dilution of clotting factors. The aging of a thrombus can be classified pathologically into the following two groups according to previously accepted definitions:10 1) a fresh thrombus (<1 day) is composed of a layered pattern of platelets, fibrin, erythrocytes, and intact granulocytes; and 2) lytic and organized thrombi (>1 day) are characterized by areas of necrosis, apoptosis, and karyorrhexis of granulocytes. In particular, thrombus age can be estimated based in the presence of intact granulocytes (average life span of 5.4 days) as well as erythrocytes. The thrombus at the second stroke in our case seemed to be a poor RBC-rich thrombus based on H&E staining, but a large RBC component remained on antiglycophorin-A staining which is known to detect the sialoglycoprotein of RBC membranes for up to 6 days in water and 15 days in air.11 These findings suggest that the size of the RBC component in the intracardiac thrombi did not alter during maturation of the thrombus in the heart. Furthermore, the first thrombus was considered a fresh one because it exhibited intact RBCs and granulocytes, while the second thrombus exhibited RBCs with an abnormal shape and granulocyte apoptosis, which suggests an older thrombus. Although there are no data regarding direct comparicomparisons between antiglycophorin-A staining and H&E staining in terms of thrombus age, our findings were suggestive of the aging process of cardiac thrombus.1011
Figures and Tables
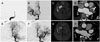 | Fig. 1Digital subtraction angiography (DSA), brain MRI, and coronary computed tomography (CT) of the patient. A: Initial DSA showing thromboembolic occlusion of the right distal internal carotid artery (ICA). B: Mechanical thrombectomy with the Solitaire (ev3 Inc.) device against the right distal ICA and middle cerebral artery (MCA), which achieved complete recanalization. C: Diffusion-weighted MRI revealing an acute infarction in the right insula and basal ganglia. D: Coronary CT demonstrating a 22-mm-sized thrombus (white arrow) in the left atrial appendage. E: The patient suffered a second stroke 6 days after the index stroke; DSA revealing embolic occlusion of the left proximal MCA. F: The left MCA was successfully reopened after mechanical thrombectomy using the suction thrombectomy and Solitaire (ev3 Inc.) devices. G: Follow-up diffusion-weighted MRI revealing a slightly increased signal intensity in the left basal ganglia. H: Follow-up coronary CT after the second stroke confirming disappearance of the thrombus in the heart. |
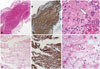 | Fig. 2Hematoxylin and eosin staining (original magnification ×40). (A) The extracted thrombus from the index stroke exhibited a large number of red blood cells (RBCs), whereas (D) the clot extracted from the second stroke appeared to have fewer RBCs and a larger amount of fibrin. However, antiglycophorin-A staining (original magnification ×40) revealed that the thrombus extracted following the first (B) and second (E) strokes were erythrocyte-rich thrombi. (C) Detail of panel A showing intact RBCs and granulocytes (white arrow). (F) Detail of panel D revealing degraded RBCs and granulocyte apoptosis (white arrowhead). |
Acknowledgements
This work was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (HI10C2020).
References
1. Layton KF, White JB, Cloft HJ, Kallmes DF, Manno EM. Expanding the treatment window with mechanical thrombectomy in acute ischemic stroke. Neuroradiology. 2006; 48:402–404.

2. Nogueira RG, Smith WS. MERCI and Multi MERCI Writing Committee. Safety and efficacy of endovascular thrombectomy in patients with abnormal hemostasis: pooled analysis of the MERCI and multi MERCI trials. Stroke. 2009; 40:516–522.

3. Nogueira RG, Yoo AJ, Buonanno FS, Hirsch JA. Endovascular approaches to acute stroke, part 2: a comprehensive review of studies and trials. AJNR Am J Neuroradiol. 2009; 30:859–875.

4. Nogueira RG, Schwamm LH, Hirsch JA. Endovascular approaches to acute stroke, part 1: Drugs, devices, and data. AJNR Am J Neuroradiol. 2009; 30:649–661.

5. Koh JS, Lee SJ, Ryu CW, Kim HS. Safety and efficacy of mechanical thrombectomy with solitaire stent retrieval for acute ischemic stroke: a systematic review. Neurointervention. 2012; 7:1–9.

6. Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008; 39:1205–1212.
7. Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003; 227:522–528.

8. Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev. 2010; 6:150–161.

9. Bernhardt P, Schmidt H, Hammerstingl C, Lüderitz B, Omran H. Atrial thrombi-a prospective follow-up study over 3 years with transesophageal echocardiography and cranial magnetic resonance imaging. Echocardiography. 2006; 23:388–394.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download