This article has been
cited by other articles in ScienceCentral.
Abstract
Background and Purpose
Acute myelitis patients exhibiting only sensory deficits upon initial presentation are not commonly encountered in clinical practice, but they definitely exist. Since acute sensory myelitis has not been investigated previously, this study evaluated the etiological spectrum of the condition with the aim of describing the clinical characteristics thereof.
Methods
Patients with acute myelitis who presented at the Ewha Womans University Medical Center (during 1999-2012) and the National Cancer Center (during 2005-2014) with only sensory symptoms as first clinical features were enrolled in this study. Their medical records, electrophysiological and laboratory data, and MRI findings were analyzed retrospectively.
Results
Of a total of 341 acute myelitis patients, 52 (15%) were identified as having acute sensory myelitis. The male-to-female ratio of these patients was 35:17, and their age at the onset of the condition was 41.7±10.5 years (mean±SD; range, 24-72 years). Acute sensory myelitis developed in patients with multiple sclerosis (MS; 14%), neuromyelitis optica spectrum disorder (NMOSD; 17%), and acute myelitis associated with concurrent systemic diseases including Behçet's disease and cancer (6%). Despite detailed evaluation, the etiology of 33 patients with acute myelitis could not be determined. Longitudinally extensive transverse myelitis on spinal MRI and progression of the sensory level were observed most commonly in NMOSD patients (89% and 78%, respectively); however, these patients did not exhibit sensory dissociation. Residual negative sensory symptoms were observed more frequently in NMOSD patients (33%) than in those with acute myelitis of unknown cause (24%) or MS (14%). During the long-term follow-up (4.7±2.7 years) of patients who did not undergo maintenance immunotherapy, a monophasic clinical course was common in those with acute myelitis of unknown cause (76%), but not in NMOSD or MS patients.
Conclusions
Accurate identification of the diverse nature of acute sensory myelitis may assist in patient care.
Keywords: myelitis, acute, sensory, etiology
INTRODUCTION
The term "myelitis" covers heterogeneous inflammatory conditions of the spinal cord that are characterized by acute-to-subacute motor, sensory, and autonomic dysfunctions, and may have various causes. Myelitis symptoms are caused by destruction of ascending or descending pathways or the gray matter of the spinal cord. Patients with myelitis present with various sensory symptoms, with a disturbance in "sensory level" being the most characteristic feature.
12
A subset of patients with acute myelitis initially presents with only sensory symptoms. Acute myelitis with sensory deficits (or "acute sensory myelitis") is relatively rare in clinical practice, but it definitely exists. However, to the best of our knowledge, detailed clinical data on such patients have not yet been described.
Detection of acute sensory myelitis requires careful neurological examination based on a clinical suspicion of the condition and a spinal cord MRI. The etiological spectrum of patients with acute myelitis who initially presented with sensory symptoms only is described herein, thus representing the first clinical issues encountered.
METHODS
Patients with acute myelitis who presented initially with only sensory symptoms at the Ewha Womans University Medical Center (EUMC) from January 1999 to June 2012 and the National Cancer Center (NCC) from September 2005 to April 2014 were enrolled. Patients who presented with sensory symptoms only, and particularly with sensory level, within 3 weeks of the nadir, and who exhibited high-level T2-weighted signals on spinal MRI were included according to the clinical judgment of physician. Sensory symptoms were divided into positive (neuropathic pain, hyperalgesia, allodynia, and paresthesia) and negative (hypesthesia and numbness) symptoms. All patients exhibiting other signs of motor or autonomic dysfunction upon careful neurological examination were excluded. Patients were examined neurologically and underwent spinal MRI within 1 month of disease onset.
The medical records, electrophysiological and laboratory data, and MRI scans of the included patients were reviewed and analyzed retrospectively. All patients with neuromyelitis optica spectrum disorder (NMOSD)
3 were positive for the aquaporin 4 (AQP4) antibody, and all multiple sclerosis (MS) patients met the McDonald criteria.
45 Patients with MS and acute myelitis of unknown cause were confirmed rigorously to be negative for serum AQP4 antibody via repeat assays using three different methods: the AQP4-M23 isoform commercial cell-based assay (CBA; Euroimmun, Luebeck, Germany),
6 an in-house CBA at Tohoku University,
7 and an in-house enzyme-linked immunosorbent assay at the NCC.
8 To ensure the idiopathic nature of acute myelitis of unknown cause, patients in whom vascular and compressive lesions were identified by spinal MRI were excluded, and patients were screened for infections and metabolic problems. After this detailed evaluation, 33 patients were ultimately diagnosed with acute myelitis of unknown etiology. Of these patients, eight were not tested for AQP4 antibody because they visited our clinic prior to the NMOSD era (2004). However, all eight patients exhibited a monophasic clinical course over a 2-year follow-up period, and no involvement of the optic nerve or brain were observed, which would have supported diagnoses of NMOSD or MS, respectively.
The Institutional Review Board of each participating center approved the study protocol and waived the need for informed consent since all of the data were anonymized.
RESULTS
Of 341 patients with acute myelitis, 52 patients (15%) satisfying the eligibility criteria for acute sensory myelitis were enrolled at the EUMC and NCC (12 of 108 EUMC patients over 13 years, and 40 of 233 NCC patients over 9 years). The age at disease onset was 41.7±10.5 years (mean±SD; range, 24-72 years), and the follow-up duration was 4.7±2.7 years (range, 2-11 years); the male-to-female ratio was 35:17. The clinical characteristics and spinal MRI findings of the acute sensory myelitis patients are listed in
Table 1.
Etiologies
The acute sensory myelitis patients had MS (
n=7, 14%), NMOSD (
n=9, 17%), or acute myelitis with concurrent systemic diseases, including Behçet's disease and cancer (breast and colon cancer;
n=3, 6%). After detailed evaluation, the etiology of the condition remained unknown for 33 patients (
Table 1).
Comparison of the characteristics of acute sensory myelitis with different etiologies
Table 2 provides the characteristics of acute sensory myelitis with different entities. The mean age of onset, and follow-up duration did not differ significantly among the groups of patients with different clinical entities. In patients not undergoing maintenance immunotherapy, a monophasic clinical course was observed exclusively in those with acute myelitis of unknown cause (76%), and thus not in those with MS or NMOSD. The two patients with concurrent cancer did not relapse during follow-up, even though they did not receive maintenance immunotherapy, rather only anticancer therapy.
There were no significant differences in sensory symptoms or signs among patients with diseases of different etiologies. However, a trend toward the development of negative sensory symptoms was evident in MS (71%) and NMOSD (78%) patients compared with those with acute myelitis of unknown cause (67%). Furthermore, the thoracic level tended to be involved preferentially in NMOSD patients (89%); ascending and descending progression of the sensory level was also observed most commonly in these patients (78%). In contrast, dissociated sensory symptoms were not noted in NMOSD patients. Asymmetric sensory involvement and a normal deep tendon reflex were common across the etiological spectrum.
In spinal MRI scans obtained during the acute phase, lesion levels did not differ significantly among patients with different disease entities. However, cervical lesions tended to be common in MS patients (71%), whereas thoracic lesions were usually observed in NMOSD patients (89%). The mean NMOSD lesion length on spinal MRI was significantly greater than that of MS patients (
p=0.036). Furthermore, longitudinally extensive transverse myelitis (LETM) extending over three vertebral segments on spinal MRI was observed more commonly among NMOSD patients (89%) than in those with MS (14%) or acute myelitis of unknown cause (52%;
p=0.019). Otherwise, axial spinal MRI revealed more peripheral lesions in MS patients than in those with the other disease entities (
p=0.002) (
Fig. 1).
Brain MRI was performed on 44 patients during follow-up. A greater proportion of MS patients had brain MRI lesions (100%) compared with patients with NMOSD (22%) or acute myelitis of unknown cause (19%; p<0.001). Evaluation of sensory evoked potentials revealed that conduction defects tended to occur more commonly in the MS patients (50%). Visually evoked potentials were assessed in 37 patients, and conduction defects were evident more frequently in NMOSD patients (40%) than in those with MS (14%) or acute myelitis of unknown cause (0%; p=0.036).
Upon cerebrospinal fluid (CSF) evaluation during the acute stage, an immunoglobulin G (IgG) index of >0.7 was observed only in MS patients (67%). No oligoclonal band was detected, but isoelectric focusing (IEF) was not performed. During follow-up, all NMOSD patients were positive for the AQP4 antibody. Autoimmune laboratory data were abnormal in 6% of all patients (3/52), antinuclear antibodies were detected in one patient with MS and one with acute myelitis of unknown cause, and the anti-Sjörgen's syndrome A antibody status was positive in one NMOSD patient.
Acute treatment with high-dose steroids (methylprednisolone; 1 g/day) was usually prescribed for patients across the etiological spectrum. Partial recovery was observed commonly after such acute treatment, and residual negative sensory symptoms were more common in NMOSD patients (33%) than in those with acute myelitis of unknown cause (24%) or MS (14%). Immunotherapy to prevent relapse was prescribed for MS and NMOSD patients, and for those with relapsing myelitis of unknown cause.
DISCUSSION
Of the acute sensory myelitis patients studied herein, some ultimately developed MS (14%), or interestingly, even NMOSD (17%), which require long-term immunotherapy. Spinal MRI revealed variations in lesion length (ranging from one to eight vertebral segments), and LETM was also observed. LETM and progression of the sensory level, which may reflect the presence of severe inflammation, were most commonly observed in NMOSD patients (89% and 78%, respectively). In contrast, NMOSD patients did not exhibit sensory dissociation. Patients commonly received steroid therapy during the acute phase (92%), and residual negative sensory symptoms, which represent disabilities, were observed more frequently in NMOSD (33%) patients than in those with acute myelitis of unknown cause (24%) or MS (14%). During the long-term follow-up (4.7±2.7 years) of patients not undergoing maintenance immunotherapy, a monophasic clinical course was common in those with acute myelitis of unknown cause (76%), but not in patients with NMOSD or MS.
Brain MRI (in 100% of MS patients), and sensory evoked potentials (in 50% of MS patients) revealing defects in the posterior column pathway were commonly observed abnormalities in MS patients. Assessment of visually evoked potentials usually revealed conduction defects in NMOSD patients (40%). IgG index abnormalities (scores of >0.7) were observed only in MS patients (67%), but no oligoclonal band was detected, probably because of a technical limitation (IEF was not performed).
9
The etiological or concurrent diseases suffered by our patients included MS, NMOSD, Behçet's disease, and cancer. After careful evaluation, the diseases of 33 patients who tended to present with monophasic clinical courses were ultimately categorized as "unknown etiology". The specific involvement of the sensory system suggests that involvement of specific tracts and immunological processes contribute to the pathogenic mechanisms of the disease. For example, some patients with paraneoplastic syndromes, such as amphiphysin autoimmunity, exhibited tract-restricted signal abnormalities on spinal MRI. Although the patient who was positive for amphiphysin antibody exhibited predominantly motor system involvement, this constitutes evidence that the immune response is anatomically confined. Thus, paraneoplastic myelopathy may be another cause of acute sensory myelitis.
10 The retrospective nature of the present study rendered it impossible to check for paraneoplastic antibodies, but two patients had concurrent breast or colon cancer. Moreover, atopic myelitis, which triggers prominent sensory symptoms with or without mild motor weakness,
1112 exhibits similar features, and may cause acute sensory myelitis. Further prospective studies with larger cohorts and longer follow-up periods may answer the question of the "unknown etiology" of acute sensory myelitis.
The retrospective nature of this study was associated with methodological shortcomings and limitations due to the participation of only two referral centers. Thus, the observed etiological spectrum may be biased by the inclusion of a relatively small proportion of MS patients, possibly due to referral bias or patient's ethnicity. In addition, some patients were not subjected to paraclinical evaluation of CSF properties and/or were not evaluated electrophysiologically. Finally, sensory recovery was estimated by the treating physicians not always using a defined scale.
The etiological spectrum of patients presenting initially with acute sensory myelitis alone was described in this study. An understanding of the diverse nature of acute sensory myelitis is important when seeking to improve the quality of life of these patients.
Figures and Tables
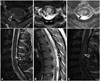 | Fig. 1High signal lesions (arrowheads) on T2-weighted spinal MRI (transverse, longitudinal section) of patients with multiple sclerosis (A), neuromyelitis optica spectrum disorder (B), and acute myelitis of unknown cause (C).
|
Table 1
Demographics and clinical findings of acute sensory myelitis (total, n=52)

|
Age at onset (years) |
41.7±10.5 (24-72) |
|
Gender ratio (male:female) |
35:17 |
|
Etiology (n, %) |
|
|
Multiple sclerosis |
7 (14) |
|
Neuromyelitis optica spectrum disorder |
9 (17) |
|
Acute sensory myelitis with unknown cause |
33 (63) |
|
Others (with systemic diseases) |
3 (6) |
|
Follow-up periods (years) |
4.7±2.7 (2-11) |
|
Monophasic clinical course (n, %) |
25 (48) |
|
Sensory symptoms (n, %) |
|
|
Sensory level |
|
|
Cervical |
17 (33) |
|
Thoracic |
26 (50) |
|
Lumbar |
9 (17) |
|
Positive or negative symptoms |
|
|
Positive |
15 (29) |
|
Negative |
6 (12) |
|
Both |
31 (59) |
|
Symmetric symptom development |
|
|
Symmetric |
13 (25) |
|
Asymmetric |
39 (75) |
|
Sensory level progression |
|
|
Ascending pattern |
18 (35) |
|
Descending pattern |
9 (17) |
|
None |
25 (48) |
|
Neurological signs (n, %) |
|
|
Sensory dissociation |
|
|
Yes (pain and temperature only) |
12/47 (26) |
|
No |
35/47 (74) |
|
Deep tendon reflex |
|
|
Normoactive |
26/40 (65) |
|
Hyperactive |
13/40 (33) |
|
Hypoactive |
1/40 (2) |
|
Lhermitte's sign |
12/17 (71) |
|
Spinal MRI (n, %) |
|
|
Lesion level |
|
|
Cervical |
20 (38) |
|
Cervicothoracic |
2 (4) |
|
Thoracic |
30 (58) |
|
Lesion length (vertebral segments) |
2.8±1.4 (1-8) |
|
Axial location |
|
|
Central |
17 (33) |
|
Peripheral |
11 (21) |
|
Both |
24 (46) |
|
Enhancement |
41/44 (93) |
|
Acute treatment (n, %) |
|
|
Steroid |
48 (92) |
|
None |
4 (8) |
|
Recovery after acute phase (n, %) |
|
|
Complete |
8 (15) |
|
Partial |
29 (56) |
|
None |
15 (29) |
|
Residual sensory symptoms after acute phase (n, %) |
|
|
Positive symptoms |
26 (50) |
|
Negative symptoms |
4 (8) |
|
Both symptoms |
9 (17) |
|
None |
13 (25) |
|
Symptomatic treatment (n, %) |
29 (56) |
|
Immunotherapy for maintenance (n, %) |
29 (56) |

Table 2
Comparison of the characteristics of the different etiologies of acute sensory myelitis

|
Multiple sclerosis (n=7) |
Neuromyelitis optica spectrum disorder (n=9) |
Acute sensory myelitis with unknown cause (n=33) |
Acute sensory myelitis with concurrent systemic diseases (n=3) |
p-value |
|
Age at onset (years) |
41±16 |
41±11 |
42±9 |
44±18 |
NS |
|
Gender ratio (female:male) |
4:3 |
8:1 |
4:29*
|
1:2 |
0.002 |
|
Follow-up (years) |
3.8±2.0 |
4.9±2.8 |
5.1±2.8 |
2.7±1.2 |
NS |
|
Monophasic course (n, %) |
|
|
|
|
|
|
Overall |
2 (29) |
1 (11) |
19 (58)*
|
3 (100) |
0.008 |
|
Without maintenance immunotherapy |
0/0 (0) |
0/0 (0) |
16/21 (76)*
|
2/2 (100) |
<0.001 |
|
Sensory symptoms (n, %) |
|
|
|
|
|
|
Positive/negative symptoms |
|
|
|
|
|
|
Positive |
2 (29) |
2 (22) |
11 (33) |
0 (0) |
NS |
|
Negative |
1 (14) |
2 (22) |
3 (9) |
0 (0) |
NS |
|
Both |
4 (57) |
5 (56) |
19 (58) |
3 (100) |
NS |
|
Sensory level |
|
|
|
|
|
|
Cervical |
3 (43) |
1 (11) |
13 (39) |
0 (0) |
NS |
|
Thoracic |
3 (43) |
8 (89) |
13 (39) |
2 (67) |
NS |
|
Lumbar |
1 (14) |
0 (0) |
7 (22) |
1 (33) |
NS |
|
Symmetry |
|
|
|
|
|
|
Symmetric |
1 (14) |
3 (33) |
7 (21) |
2 (67) |
NS |
|
Asymmetric |
6 (86) |
6 (67) |
26 (79) |
1 (33) |
NS |
|
Level progression |
|
|
|
|
|
|
Ascending |
3 (43) |
3 (33) |
11 (33) |
1 (33) |
NS |
|
Descending |
0 (0) |
4 (45) |
5 (15) |
0 (0) |
NS |
|
None |
4 (57) |
2 (22) |
17 (52) |
2 (67) |
NS |
|
Neurological signs (n, %) |
|
|
|
|
|
|
Sensory dissociation |
|
|
|
|
|
|
Yes |
2/6 (33) |
0/7 (0) |
10/31 (32) |
0/3 (0) |
NS |
|
No |
4/6 (67) |
7/7 (100) |
21/31 (68) |
3/3 (100) |
NS |
|
Deep tendon reflex |
|
|
|
|
|
|
Normoactive |
3/5 (60) |
4/5 (80) |
18/27 (67) |
1/3 (33) |
NS |
|
Hyperactive |
2/5 (40) |
1 (20) |
8/27 (30) |
2/3 (67) |
NS |
|
Hypoactive |
0 (0) |
0 (0) |
1/27 (3) |
0 (0) |
NS |
|
Spinal MRI (n, %) |
|
|
|
|
|
|
Lesion level |
|
|
|
|
|
|
Cervical |
4 (57) |
1 (11) |
15 (46) |
0 (0) |
NS |
|
Cervicothoracic |
1 (14) |
0 (0) |
1 (3) |
0 (0) |
NS |
|
Thoracic |
2 (29) |
8 (89) |
17 (51) |
3 (100) |
NS |
|
Lesion length |
1.9±1.0*
|
3.7±1.1*
|
2.6±1.1 |
3.6±3.8 |
0.036 |
|
LETM |
1/7 (14) |
8/9 (89)*
|
17/33 (52) |
1/3 (33) |
0.019 |
|
Axial location |
|
|
|
|
|
|
Central |
0 (0) |
4 (44) |
11 (33) |
2 (67) |
0.002 |
|
Peripheral |
6 (86)*
|
0 (0) |
5 (15) |
0 (0) |
0.002 |
|
Both |
1 (14) |
5 (56) |
17 (52) |
1 (33) |
0.002 |
|
Enhancement |
5/6 (83) |
8/8 (100) |
26/27 (96) |
2/3 (67) |
NS |
|
Brain MRI lesions (n, %) |
7/7 (100)*
|
2/9 (22) |
5/26 (19) |
0/2 (0) |
<0.001 |
|
Abnormality on SEPs (n, %) |
3/6 (50) |
0/2 (0) |
2/15 (13) |
0/1 (0) |
NS |
|
Abnormality on VEPs (n, %) |
1/7 (14) |
2/5 (40)*
|
0/23 (0) |
0/2 (0) |
0.036 |
|
CSF (n, %) |
|
|
|
|
|
|
Pleocytosis |
1/5 (20) |
2/8 (25) |
5/32 (16) |
0/2 (0) |
NS |
|
Increased protein |
1/5 (20) |
1/7 (14) |
6/32 (19) |
0/2 (0) |
NS |
|
IgG index >0.7 |
4/6 (67)*
|
0/7 (0) |
0/27 (0) |
0/1 (0) |
0.002 |
|
Oligoclonal band |
0/4 (0) |
0/8 (0) |
2/22 (9) |
0/1 (0) |
NS |
|
Serum (n, %) |
|
|
|
|
|
|
Aquaporin-4 antibody |
0/7 (0) |
9/9 (100)*
|
0/25 (0) |
0/2 (0) |
<0.001 |
|
Autoimmune antibodies |
1/7 (14) |
1/9 (11) |
1/33 (3) |
0/3 (0) |
NS |
|
Antinuclear antibody |
1 |
0 |
1 |
0 |
NS |
|
Anti-SSA antibody |
0 |
1 |
0 |
0 |
NS |
|
Acute treatment (n, %) |
|
|
|
|
|
|
Steroid |
7 (100) |
8 (89) |
30 (91) |
3 (100) |
NS |
|
None |
0 (0) |
1 (11) |
3 (9) |
0 (0) |
NS |
|
Recovery after acute treatment (n, %) |
|
|
|
|
|
|
Complete |
3 (42) |
1 (11) |
8 (24) |
0 (0) |
NS |
|
Partial |
2 (29) |
7 (78) |
14 (43) |
2 (67) |
NS |
|
None |
2 (29) |
1 (11) |
11 (33) |
1 (33) |
NS |
|
Residual sensory symptoms after acute treatment (n, %) |
|
|
|
|
|
|
Positive |
3 (43) |
5 (56) |
17 (52) |
2 (67) |
NS |
|
Negative |
0 (0) |
2 (22) |
2 (6) |
0 (0) |
NS |
|
Both |
1 (14) |
1 (11) |
6 (18) |
1 (33) |
NS |
|
None |
3 (43) |
1 (11) |
8 (24) |
0 (0) |
NS |
|
Symptomatic treatment (n, %) |
4 (57) |
5 (56) |
19 (58) |
1 (33) |
NS |
|
Immunotherapy (n, %) |
7 (100)*
|
9 (100)*
|
12 (36) |
1 (33) |
<0.001 |








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download