Abstract
Neurocysticercosis is an infection of the central nervous system caused by the larval form of the pork tapeworm Taenia solium. In the brain it occurs in two forms: parenchymal and extraparenchymal or racemose cysts. The clinical presentation of racemose cysts is pleomorphic, and is quite different from parenchymal cysticercosis. The clinical diagnosis of racemose cysts is quite challenging, with neuroimaging being the mainstay. However, the advent of newer brain imaging modalities has made a more accurate diagnosis possible. The primary focus of this article is racemose neurocysticercosis and its multitude manifestations, and includes a discussion of the newer diagnostic modalities and treatment options.
Cysticercosis is a zoonosis caused by the encysted larval stage of the tapeworm Taenia solium. Cysticercus refers to the resting stage of the larva of the cestodes. It can affect any organ of the body, but the cerebral parenchyma is the most common site in the central nervous system (CNS; 60-90%), where it causes neurocysticercosis. Neurocysticercosis is one of the most common helminthic human infestations of the CNS1 and is one of the most common causes of acquired seizures in endemic countries.2 Cysticercosis is highly endemic in most developing countries because of poor socioeconomic development.3 About 50 million people in the world today are infected by the taeniasis-cysticercosis complex, and 50,000 of those die every year as a result.4 Human infestation develops after the ingestion of eggs from the feces of a tapeworm carrier (i.e., fecal-oral contamination) or consumption of meat from an intermediate host.5 In the brain, it occurs in two forms: parenchymal and extraparenchymal or racemose cysts. The clinical presentation of racemose cysts is pleomorphic, and quite different from parenchymal cysticercosis.
Humans are the only definite hosts for T. solium, while pigs act as the intermediate hosts. Humans can also act as intermediate hosts after consumption of T. solium eggs. In the intermediate host, it transforms into a "bladder worm" composed of a vesicle with a central fluid-containing cavity and an invaginated scolex on its wall. In the human host, adult tapeworms develop in the intestine after ingesting cysticercus in undercooked pork. These adult tapeworms shed proglottids, with each proglottid containing 1,000-2,000 eggs. The oncospheres hatch in the intestine and then penetrate the intestinal wall before disseminating to various body tissues including the CNS to form cysticerci. The cysticerci in the brain undergo four stages of involution.6 Stage one is the vesicular stage, which is characterized by a cyst with a vesicular, translucent wall with transparent fluid and a viable invaginated scolex. This stage is followed by the colloidal stage, which is characterized by a cyst with a thick wall, turbid fluid, and a degenerating scolex and induces a host inflammatory response. The cyst subsequently degenerates as it moves into the granular stage, which is characterized by a cyst with a thicker wall, degenerated scolex, and little associated inflammatory response. Finally, the cyst transforms into a coarse calcified nodule (calcific stage).
There are several types of neurocysticercosis lesion based on the location of the parasite in the CNS. They frequently present as focal lesions in the brain parenchyma with surrounding edema, which subsequently undergo calcification. The most frequent CNS location is in the cerebral hemispheres, commonly at the junction between the gray and white matters.7 Cysticercosis in a cerebellar location is rare, but there have been a few case reports.89 The rarity of cysticercosis in the cerebellum may be attributable to its less-abundant blood flow relative to the cerebrum.
Other extraparenchymal CNS sites include the ventricles, cisternal/subarachnoid spaces, spinal cord, and ocular bulb.10 Many patients may have a combination of these types. Cysts in a subarachnoid location are less common and occur in the basal cisterns or Sylvian fissures. Macroscopically, they appear like a cluster of grapes and are termed a racemose variety of neurocysticercosis.11 Racemose neurocysticercosiss differ from parenchymal lesions in several ways, and are referred to as "aberrant proliferating cestode larvae." They arise from segmentation of cysticercosis cellulosae with sprouting of new cysts. These new cysts gradually expand with degeneration of the scolex and are nonviable and degenerated interconnected bladders of various sizes.12 They are seen as abnormal growths of cystic membranes without a scolex that occur in and around the brain in areas such as the suprasellar, Sylvian, and quadrigeminal cisterns. They are larger because in the cisternal or subarachnoid space there is no limiting parenchymal tissue that can act as a host response for encapsulation. The size of racemose neurocysticercosises varies from 4 to 12 cm,13 and they are identified on magnetic resonance (MR) imaging (MRI) as multiple cystic lesions.
The usual temporal development of the degenerative stages as seen in the aforementioned parenchymal types is lacking in racemose types,14 and the scolices on its wall are absent in most racemose cases (Fig. 1).15 Racemose cysticercus may be due to a different variety of cestode, which could be either an aberrant cysticercus of T. solium or a sterile coenurus of Taenia multiceps or Taenia serialis.16 It is clinically more aggressive than the parenchymatous form and is reportedly seen in 15-54% of patients.17181920 Racemose neurocysticercosises are considered as malignant forms of neurocysticercosis if they are associated with hydrocephalus secondary to cysticercal meningitis, and are associated with a high mortality rate (50%).21 Giant neurocysticercosis is defined as neurocysticercosises with a largest dimension of more than 4 or 5 cm.22 Racemose neurocysticercosises are rarely seen in India.23
Racemose neurocysticercosis represents cysticercal decay with cyst enlargement and hydropic changes.24 Racemose cysticerci do not usually contain scolices and are considered nonviable encysted organisms. The degenerated vesicle wall is characteristically convoluted with external bulbous projections, and evokes an inflammatory response. The associated reactive process can also cause meningitis or arteritis.25
Racemose neurocysticercosis usually presents as a meningeal, intraventricular, or subarachnoid (cisternal) form (Figs. 2 and 3). The meningeal form presents with raised intracranial pressure due to various causes. It causes widespread meningitis and adhesions that result in cerebrospinal fluid (CSF) obstruction and hydrocephalus. It may subsequently cause vasculitis and entrapment of the cranial nerves in the inflammatory exudate, resulting in a focal neurological deficit.2627 Entrapment of oculomotor nerves may cause extraocular muscle paralysis and diplopia. The optic nerve and optic chiasm may become encased within the exudate, causing decreased vision and visual field defects.2829 In the intraventricular and subarachnoid (cisternal) forms, the oncospheres reach the ventricles via the choroid plexus. These parasites occlude the CSF pathway causing acute episodes of ventriculomegaly and a mass effect. Death of the larva causes ependymitis, which cause ventricular outlet obstruction and hydrocephalus.30 Patients present with raised intracranial pressure due to hydrocephalus secondary to basal meningitis and ependymitis.19 Ependymitis may cause ventricular entrapment, causing double-compartment syndrome.18
Neurocysticercosis, and in particular the subarachnoid forms, may produce cerebrovascular disease including cerebral infarction, transient ischemic attacks, and cerebral hemorrhage.3132 The most common underlying mechanism is the induction of arteritis.3334 Barinagarrementeria et al.35 found that 54% of 28 patients with subarachnoid forms of neurocysticercosis had angiographic evidence of cerebral arteritis. They reported that the middle cerebral and posterior cerebral arteries were the most commonly involved cerebral vessels associated with clinical stroke syndromes.35 Middle-sized cerebral blood vessel involvement is a common finding in subarachnoid forms of neurocysticercosis. Small- or large-vessel involvement may also cause infarcts in subarachnoid neurocysticercosises.36 Three cases of stroke secondary to neurocysticercosis were reported by Rocha et al.37: one patient had stroke secondary to subarachnoid cysts, causing bilateral middle cerebral artery occlusion that was angiographically proven; the second patient had arteritis of the basilar and carotid arterial systems secondary to cisternal cysts; and the third patient had vasculitis of the small cortical vessels.
The intraventricular form of neurocysticercosis requires prompt, aggressive intervention in view of their rapidly progressive clinical course, and is often associated with a poor prognosis. It may occur along with the parenchymatous form or (more commonly) in isolation. The fourth ventricle is the most-common site (53%), followed by the third ventricle (27%), lateral ventricle (11%), and the aqueduct (9%).17 Mobile cysts within the lateral ventricle migrate toward the fourth ventricle due to the effects of gravity. They become entrapped in the fourth ventricle due to the size of the foramina of Magendie and Luschka. Patients with lateral ventricle cysticerci suffer from increased intracranial pressure with compression of the adjacent structures.3839 Patients with third-ventricular cysticerci present with sudden loss of consciousness due to acute hydrocephalus or progressively worsening headache and vomiting.1318 Cysts in the fourth ventricle present with subacute hydrocephalus with signs of brainstem dysfunction.40 Fourth ventricle cysts can also induce Bruns' syndrome, which is characterized by episodic headache, vomiting, papilledema, neck stiffness, and sudden positional vertigo induced by rotatory movements of the head, drop attacks, and loss of consciousness with rapid recovery. Rodriguez et al.41 reported a similar syndrome due to racemose neurocysticercosis involving the lateral, third, and fourth ventricles, basal cistern, and pineal region (Figs. 4 and 5). The exact pathomechanism of Bruns' syndrome remains unclear. The symptoms may occur due to episodic elevation of intracranial pressure as a result of changes in the cyst position, with a change in head posture causing blocking of the ventricular outflow via a ball-valve mechanism.4243
In rare cases racemose neurocysticercosis can cause dementia as a sole presenting feature in the patient.44 However, 66-87.5% of patients with neurocysticercosis have been reported to have cognitive disturbances, and dementia or severe cognitive decline has been reported in 12.5-15.6% of patients.454647 The mechanisms proposed for cognitive decline in neurocysticercosis include raised intracranial pressure, number and location of neurocysticercosises, different phases of evolution, inflammatory cytokines, and the host's immune response.45 The reversibility of dementia depends upon the predominant underlying mechanism. Dementia due to raised intracranial pressure as an underlying mechanism has an excellent outcome as compared to multiple parenchymal parasitic and vascular lesions. Racemose neurocysticercosis can also present with a mass effect (Figs. 6 and 7).48 There are reports of racemose neurocysticercosis occurring in the cerebellar hemisphere.49
Involvement of the spinal cord in neurocysticercosis is rare, reportedly accounting for 1-5% of all cases of neurocysticercosis.50 Spinal neurocysticercosis can present as leptomeningeal (extramedullary) or intramedullary forms, with the former being six to eight times more common than the latter. They are the extension of a cerebral subarachnoid disease to the spinal subarachnoid space. Cysts may be singular or may form into clumps of several cysts distributed throughout the spinal subarachnoid space.5152 The intramedullary form occurs commonly in the thoracic spinal cord and is secondary to hematogenous or ventriculoependymal migration.5354 Spinal leptomeningeal neurocysticercosis is difficult to treat, especially if it is associated with arachnoiditis, and has a poor prognosis.55 The clinical manifestations of racemose neurocysticercosis are listed in Table 1.
Computed tomography (CT) of the head has a sensitivity and specificity of more than 95% in the diagnosis of the parenchymatous form of neurocysticercosis.5657 However, it has lower sensitivity and specificity for diagnosing the ventricular and cisternal forms. These scans are helpful in detecting calcifications. Parenchymal forms of neurocysticercosis appear as single or multiple rounded lesions of low density with an eccentric mural nodule representing the scolex. The administration of contrast agent results in ring enhancement of the wall of the cyst, reflecting an inflammatory reaction. The intraventricular cysts produce dilatation of the ventricles depending on their location. Ventricular or periventricular enhancement suggests ependymitis. The presence of a ventricular cyst can be distinguished from inflammatory ventricular obstruction by positive contrast ventriculography. The presence of a regular, rounded filling defect similar to an inverted cup suggests the presence of a cyst, whereas a cul-de-sac suggests inflammatory ventricular obstruction.58 Intraventricular cysts are difficult to detect via brain CT. They appear isodense with the CSF and have thin walls, and therefore are not well visualized.
The cisternal or ventricular forms of neurocysticercosis are easily missed in CT scans, but are readily visualized in MRI. The subarachnoid cysts over the convexity of the cerebral hemispheres are small, but those in the Sylvian fissure may reach 50 mm or more in size. MRI is suitable for the identification of small parenchymal cysts-those located in the base of the brain, ventricles, and spine.10 It allows assessment of the degree of infection, the location, and the stage of the cyst in the brain.59 MRI is helpful for identifying leptomeningeal enhancement at the base of the brain secondary to arachnoiditis in cases of subarachnoid cysts.10 Intraventricular cysts have attenuation and signal intensity values similar to the CSF, and the cystic wall is very thin.6061 Conventional MR sequences such as T1- and T2-weighted images and fluid-attenuated inversion recovery images may miss intraventricular/cisternal neurocysticercosis cysts.62 The optimal MR protocol for identification of intraventricular cysts and scolex is controversial. Three-dimensional (3D), very heavily T2-weighted sequences, such as constructive interference in steady state or fast imaging employing steady-state acquisition, are good sequences for evaluating intraventricular cysts.62 These MR sequences have a high spatial resolution and signal-to-noise ratio. Another MR sequence that is also useful is the 3D spoiled gradient recalled echo sequence,63 which provides T1 information. Intraventricular cysts may also move within the ventricular cavities in relation to head movement (ventricular migration sign).64
Serological tests should detect antibodies specific for T. solium antigens for the clinical diagnosis of neurocysticercosis. The current test of choice is electroimmunotransfer blotting, which has a sensitivity of 94-98% and a specificity of 100% for patients with two or more cysts or enhanced lesions.6566 However, the sensitivity is lower in patients with solitary enhanced or calcified lesions.67 Another test is the enzyme-linked immunosorbent assay (ELISA), which is neither sensitive nor specific.68 Along with antibody assays, circulating parasite antigen assays are available that reflect the presence of live parasite and may aid in quantitative verification of successful treatment.697071 Ag-ELISA based on the monoclonal antibody reacting with a repetitive carbohydrate epitope of secretory/excretory and surface antigens of live cysticerci has been applied.72 This assay has a sensitivity of 86% and a specificity of 96%,73 and has been used for evaluating the treatment response for subarachnoid cysts in a small number of patients.74 This antigen assay should be used to follow up patients with subarachnoid cysts. Serological tests should be used in conjunction with clinical and radiological data.75
The treatment modalities available in patients with neurocysticercosis include medical management with cysticidal agents, corticosteroids, and antiepileptic drugs, and surgical management to remove the cyst or to insert a shunt to treat hydrocephalus.
There has been controversy as to whether cysticidal drugs modify the natural history of neurocysticercosis. There are arguments against the use of cysticidal drugs: 1) the cysts usually die within a short time interval and treatment may be unnecessary,76 2) scarring due to increased inflammation following initiation of drugs may worsen the long-term prognosis of seizures,77 and 3) increased inflammation due to death of the cysts. According to Salinas and Prasad.78 there is insufficient evidence to assess the beneficial effects of cysticidal drugs in neurocysticercosis.
Praziquantel and albendazole are cysticidal drugs that are effective in the treatment of all forms of neurocysticercosis.7980 Praziquantel is an isoquinolone that causes spastic paralysis of the parasite, while albendazole is an imidazole that acts by inhibiting glucose uptake by the parasites, causing energy depletion. There are no available controlled trials on the management of subarachnoid cysts. Cysticidal drugs are applied for longer when treating giant cysts and subarachnoid cysts than when treating parenchymal neurocysticercosises.2 The disappearance of intraventricular and subarachnoid cysts with cysticidal drug therapy has been reported.81 The administration of albendazole at a higher dose (30 mg/kg/day) than usual has been found to increase the clearance of intraventricular and subarachnoid cysts.82 In the treatment of intraventricular and subarachnoid cysts, a ventriculoperitoneal shunt must be placed in patients with significant obstructive hydrocephalus.83 Steroids should be given before initiating cysticidal drugs in patients with intraventricular and subarachnoid cysts in order to reduce the inflammation and the risk of shunt obstruction.84 Oral prednisolone should be given 2-3 days before initiating cysticidal drugs and continued for at least 7-10 days. In a study of 33 patients with giant subarachnoid cysts treated with albendazole (15 mg/kg/day for 4 weeks), Proaño et al.83 found that several courses of therapy were required for effective treatment. Methotrexate has been used as a steroid-sparing agent in patients with subarachnoid disease requiring long-term steroids.85
Racemose cysts in the basal cisterns of the brain cause symptoms secondary to local compression. They are usually associated with local adhesions secondary to inflammation. Excision of cysts is not recommended due to the possible presence of severe adhesions.
The surgical indications for excision are a mass effect, obstructive hydrocephalus, fourth ventricular cyst, and uncertain diagnosis. The surgical resection of ventricular cysts is by microsurgery and/or endoscopy. Acute hydrocephalus due to ventricular cysts mandates an emergency ventriculostomy to relieve the raised intracranial pressure, followed by cyst resection. A ventriculoperitoneal shunt must be placed if there is associated ependymitis. Shunt failure rates are reportedly as high as 30-70%. As a result, patients may require multiple shunt revisions, which are associated with a high mortality rate.408687 Microsurgical excision of fourth-ventricular cysts via a posterior fossa craniotomy, and supratentorial open or stereotactic craniotomy for lateral- or third-ventricular cyst excision have been described.88 However, endoscopic approaches have more recently become the treatment of choice.899091929394 The advantages of the neuroendoscopic approach include easy navigation within the ventricles and the ability to excise multiple cysts with minimal postoperative complications.9596 However, the potential limitations of the neuroendoscopic approach include intraventricular bleeding, and this procedure may be hazardous in the presence of ependymitis and dense adhesions.97 Patients with intraventricular cysts with significant ependymal enhancement are thus poor candidates for the neuroendoscopic approach. It has been reported that aspiration of the cyst contents prior to its removal in order to collapse it makes the cyst easier to handle and excise.98
Neurocysticercosis is the most common parasitic disease of the CNS and is caused by the metacestode larva of the pork tapeworm, T. solium. In the CNS, the cysticerci can present as either a parenchymal form (cysticercus cellulosae) and/or an extraparenchymal form, as racemose cysts in the subarachnoid spaces, basal cisterns, and within the ventricles. Racemose cysts are a less frequent presentation of neurocysticercosis. The presentation and management of the two forms is different. They present with raised intracranial pressure secondary to hydrocephalus, mass effect, meningitis, and vasculitis. Other manifestations include Bruns' syndrome and dementia. The investigation of choice for the diagnosis of racemose cysts is MRI. Due to its multiform manifestation, less frequent occurrence, and chances of false negativity on imaging, a high index of suspicion for the diagnosis is mandated, especially in endemic areas.
References for this review were identified by searches of the PubMed database from 1952 to September 2013, using the terms "Racemose neurocysticercosis," "neurocysticercosis," and "intraventricular cysticercus." Additional references (including abstracts) and book chapters cited in relevant articles were also reviewed. Most papers used in this review were published in English, although non-English articles with English abstracts were included when relevant.
Figures and Tables
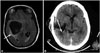 | Fig. 1A: Brain fluid-attenuated inversion recovery MRI sequence showing a hypointense cystic lesion without a scolex in the right temporal lobe, causing a mass effect (arrow). B: Brain computed tomography showing a hypodense lesion in the right perisylvian region, with a mass effect. |
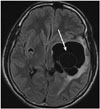 | Fig. 2Brain fluid-attenuated inversion recovery MRI sequence showing a hypointense, lobulated cystic lesion with internal septation and without a scolex in the left temporoparietal region, causing a mass effect. |
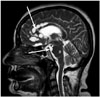 | Fig. 3Brain sagittal T2-weighted image showing a lobulated, hyperintense cystic lesion in the anterior pericallosal space and prepontine cistern (arrows). |
 | Fig. 4A: Brain coronal T1-weighted images showing a hypointense, lobulated cystic lesion with internal septation in the left Sylvian fissure (arrow). B: Brain coronal T2-weighted images showing a hyperintense, lobulated cystic lesion with internal septation in the left Sylvian fissure (arrow). |
 | Fig. 5Brain sagittal T1-weighted image showing multiple, hypointense, and cystic lesions of varying size. Some cystic lesions possess a scolex (arrow). |
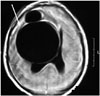 | Fig. 6Brain axial T1-weighted image showing a large, hypointense, lobulated, and cystic lesion in the right frontal lobe, causing a mass effect. |
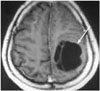 | Fig. 7Brain magnetic resonance imaging. Postcontrast axial T1-weighted image showing a hypointense cystic lesion with internal septations and no contrast enhancement. |
Table 1
Clinical manifestations of racemose neurocysticercosis

References
1. White AC Jr. Neurocysticercosis: updates on epidemiology, pathogenesis, diagnosis, and management. Annu Rev Med. 2000; 51:187–206.

2. García HH, Evans CA, Nash TE, Takayanagui OM, White AC Jr, Botero D, et al. Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev. 2002; 15:747–756.

3. Garcia HH, Del Brutto OH. Cysticercosis Working Group in Peru. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005; 4:653–661.

4. Takayanagui OM, Leite JP. [Neurocysticercosis]. Rev Soc Bras Med Trop. 2001; 34:283–290.
5. Garcia HH, Gonzales AE, Tsang VC, Gilman RH. The Cysticercosis Working Group in Peru Neurocysticercosis: some of the essentials. Pract Neurol. 2006; 6:288–297.
6. Escobar A. The pathology of neurocysticercosis. In : Palacios E, Rodriguez-Carbajal J, Taveras JM, editors. Cysticercosis of the Central Nervous System. Springfield, IL: Charles C. Thomas;1983. p. 27–54.
7. Rocca U, Rosell A, Alvarez C. Surgical options in neurocysticercosis therapy. Neurosurg Q. 2005; 15:5–13.

8. Kim JH, Suh SI, Kim JH, Kwon TH, Chung HS. Giant neurocysticercosis cyst in the cerebellar hemisphere. Neurol Med Chir (Tokyo). 2006; 46:412–414.
9. Zhu L, Shi Y, Pan X, Mo L, Weng X. Successful treatment of isolated cerebellar cysticercosis with albendazole. Chin Med J (Engl). 2003; 116:637–638.
10. Martinez HR, Rangel-Guerra R, Elizondo G, Gonzalez J, Todd LE, Ancer J, et al. MR imaging in neurocysticercosis: a study of 56 cases. AJNR Am J Neuroradiol. 1989; 10:1011–1019.
11. Khandelwal S, Sakhi P, Sharma GL, Saxena UD. Intraventricular cysticerus. Indian J Radiol Imaging. 2002; 12:329–332.
12. Rabiela Cervantes MT, Rivas-Hernandez A, Rodrigues-Ibarra J, Castillo-Medina S, Cancino FM. Anatomopathological aspects of human brain cysticercosis. In : Flisser A, Sillms K, Laclette JP, Larralde C, editors. Cysticercosis: present state of knowledge and perspectives. New York: Academic Press;1982. p. 179–200.
13. Couldwell WT, Zee CS, Apuzzo ML. Definition of the role of contemporary surgical management in cisternal and parenchymatous cysticercosis cerebri. Neurosurgery. 1991; 28:231–237.

14. Hauptman JS, Hinrichs C, Mele C, Lee HJ. Radiologic manifestations of intraventricular and subarachnoid racemose neurocysticercosis. Emerg Radiol. 2005; 11:153–157.

15. Bickerstaff ER, Cloake PC, Hughes B, Smith WT. The racemose form of cerebral cysticercosis. Brain. 1952; 75:1–18.

16. Jung RC, Rodriguez MA, Beaver PC, Schenthal JE, Levy RW. Racemose cysticercus in human brain. A case report. Am J Trop Med Hyg. 1981; 30:620–624.
17. Sinha S, Sharma BS. Neurocysticercosis: a review of current status and management. J Clin Neurosci. 2009; 16:867–876.

18. Apuzzo ML, Dobkin WR, Zee CS, Chan JC, Giannotta SL, Weiss MH. Surgical considerations in treatment of intraventricular cysticercosis. An analysis of 45 cases. J Neurosurg. 1984; 60:400–407.
19. Lobato RD, Lamas E, Portillo JM, Roger R, Esparza J, Rivas JJ, et al. Hydrocephalus in cerebral cysticercosis. Pathogenic and therapeutic considerations. J Neurosurg. 1981; 55:786–793.
20. Zee CS, Segall HD, Destian S, Ahmadi J, Apuzzo ML. MRI of intraventricular cysticercosis: surgical implications. J Comput Assist Tomogr. 1993; 17:932–939.
21. Takayanagui OM, Odashima NS. Clinical aspects of neurocysticercosis. Parasitol Int. 2006; 55:Suppl. S111–S115.

22. Colli BO, Martelli N, Assirati JA Jr, Machado HR, de Vergueiro Forjaz S. Surgical treatment of cysticercosis of the central nervous system. Neurosurg Q. 1995; 5:34–35.

23. Ghosh D, Dubey TN, Prabhakar S. Brain parenchymal, subarachnoid racemose, and intraventricular cysticercosis in an Indian man. Postgrad Med J. 1999; 75:164–166.

26. Sotelo J, Marin C. Hydrocephalus secondary to cysticercotic arachnoiditis. A long-term follow-up review of 92 cases. J Neurosurg. 1987; 66:686–689.
27. Joubert J. Cysticercal meningitis--a pernicious form of neurocysticercosis which responds poorly to praziquantel. S Afr Med J. 1990; 77:528–530.
28. Keane JR. Cysticercosis: unusual neuro-ophthalmologic signs. J Clin Neuroophthalmol. 1993; 13:194–199.
29. Keane JR. Neuro-ophthalmologic signs and symptoms of cysticercosis. Arch Ophthalmol. 1982; 100:1445–1448.

30. Stern WE. Neurosurgical considerations of cysticercosis of the central nervous system. J Neurosurg. 1981; 55:382–389.

31. Barinagarrementeria F, Del Brutto OH. Lacunar syndrome due to neurocysticercosis. Arch Neurol. 1989; 46:415–417.

32. Soto-Hernandez JL, Gomez-Llata Andrade S, Rojas-Echeverri LA, Texeira F, Romero V. Subarachnoid hemorrhage secondary to a ruptured inflammatory aneurysm: a possible manifestation of neurocysticercosis: case report. Neurosurgery. 1996; 38:197–199. discussion 199-200

33. Rodriguez-Carbajal J, Del Brutto OH, Penagos P, Huebe J, Escobar A. Occlusion of the middle cerebral artery due to cysticercotic angiitis. Stroke. 1989; 20:1095–1099.

34. Sawhney IM, Singh G, Lekhra OP, Mathuriya SN, Parihar PS, Prabhakar S. Uncommon presentations of neurocysticercosis. J Neurol Sci. 1998; 154:94–100.

35. Barinagarrementeria F, Cantú C. Frequency of cerebral arteritis in subarachnoid cysticercosis: an angiographic study. Stroke. 1998; 29:123–125.

36. Hawk MW, Shahlaie K, Kim KD, Theis JH. Neurocysticercosis: a review. Surg Neurol. 2005; 63:123–132. discussion 132

37. Rocha MS, Brucki SM, Ferraz AC, Piccolo AC. [Cerebrovascular disease and neurocysticercosis]. Arq Neuropsiquiatr. 2001; 59:778–783.
38. Jankowski R, Zimmerman RD, Leeds NE. Cysticercosis presenting as a mass lesion at foramen of Monro. J Comput Assist Tomogr. 1979; 3:694–696.

39. King JS, Hosobuchi Y. Cysticercus cyst of the lateral ventricle. Surg Neurol. 1977; 7:125–129.
40. Cuetter AC, Andrews RJ. Intraventricular neurocysticercosis: 18 consecutive patients and review of the literature. Neurosurg Focus. 2002; 12:e5.

41. Diehl Rodriquez R, Crestani DN, Dworzecki Soares JO, Franceshini PR, Petersen Alves R, Zimerman R, et al. Bruns' syndrome and racemose neurocysticercosis: a case report. Rev Soc Bras Med Trop. 2012; 45:269–271.

42. Torres-Corzo J, Rodriguez-della Vecchia R, Rangel-Castilla L. Bruns syndrome caused by intraventricular neurocysticercosis treated using flexible endoscopy. J Neurosurg. 2006; 104:746–748.

43. Krasnianski M, Müller T, Stock K, Zierz S. Bruns syndrome caused by intraventricular tumor. Eur J Med Res. 2008; 13:179–181.
44. Sharma S, Modi M, Lal V, Prabhakar S, Bhardwaj A, Sehgal R. Reversible dementia as a presenting manifestation of racemose neurocysticercosis. Ann Indian Acad Neurol. 2013; 16:88–90.

45. Ciampi de Andrade D, Rodrigues CL, Abraham R, Castro LH, Livramento JA, Machado LR, et al. Cognitive impairment and dementia in neurocysticercosis: a cross-sectional controlled study. Neurology. 2010; 74:1288–1295.

46. Ramirez-Bermudez J, Higuera J, Sosa AL, Lopez-Meza E, Lopez-Gomez M, Corona T. Is dementia reversible in patients with neurocysticercosis? J Neurol Neurosurg Psychiatry. 2005; 76:1164–1166.

47. Forlenza OV, Filho AH, Nobrega JP, dos Ramos Machado L, de Barros NG, de Camargo CH, et al. Psychiatric manifestations of neurocysticercosis: a study of 38 patients from a neurology clinic in Brazil. J Neurol Neurosurg Psychiatry. 1997; 62:612–616.

48. Kumar S, Thakur S, Jhobta A, Sood RG. Giant racemose neurocysticercosis with mass effect: unusual presentation. Ann Indian Acad Neurol. 2013; 16:398–399.

49. Kim SW, Kim MK, Oh SM, Park SH. Racemose cysticercosis in the cerebellar hemisphere. J Korean Neurosurg Soc. 2010; 48:59–61.

50. Ahmad FU, Sharma BS. Treatment of intramedullary spinal cysticercosis: report of 2 cases and review of literature. Surg Neurol. 2007; 67:74–77. discussion 77

51. Bandres JC, White AC Jr, Samo T, Murphy EC, Harris RL. Extraparenchymal neurocysticercosis: report of five cases and review of management. Clin Infect Dis. 1992; 15:799–811.

52. De Souza Queiroz L, Filho AP, Callegaro D, De Faria LL. Intramedullary cysticercosis. Case report, literature review and comments on pathogenesis. J Neurol Sci. 1975; 26:61–70.
53. Sharma BS, Banerjee AK, Kak VK. Intramedullary spinal cysticercosis. Case report and review of literature. Clin Neurol Neurosurg. 1987; 89:111–116.
54. Corral I, Quereda C, Moreno A, López-Vélez R, Martínez-San-Millán J, Guerrero A, et al. Intramedullary cysticercosis cured with drug treatment. A case report. Spine (Phila Pa 1976). 1996; 21:2284–2287.
55. Canas NM, Calado SL, Vale J. [Treatment of racemose neurocysticercosis of the spine]. Rev Neurol. 2005; 40:544–547.
56. Wadia RS, Makhale CN, Kelkar AV, Grant KB. Focal epilepsy in India with special reference to lesions showing ring or disc-like enhancement on contrast computed tomography. J Neurol Neurosurg Psychiatry. 1987; 50:1298–1301.

57. Nash TE, Neva FA. Recent advances in the diagnosis and treatment of cerebral cysticercosis. N Engl J Med. 1984; 311:1492–1496.

58. Salazar A, Sotelo J, Martinez H, Escobedo F. Differential diagnosis between ventriculitis and fourth ventricle cyst in neurocysticercosis. J Neurosurg. 1983; 59:660–663.

59. Wallin MT, Kurtzke JF. Neurocysticercosis in the United States: review of an important emerging infection. Neurology. 2004; 63:1559–1564.

61. do Amaral LL, Ferreira RM, da Rocha AJ, Ferreira NP. Neurocysticercosis: evaluation with advanced magnetic resonance techniques and atypical forms. Top Magn Reson Imaging. 2005; 16:127–144.
62. Govindappa SS, Narayanan JP, Krishnamoorthy VM, Shastry CH, Balasubramaniam A, Krishna SS. Improved detection of intraventricular cysticercal cysts with the use of three-dimensional constructive interference in steady state MR sequences. AJNR Am J Neuroradiol. 2000; 21:679–684.
63. Robbani I, Razdan S, Pandita KK. Diagnosis of intraventricular cysticercosis by magnetic resonance imaging: improved detection with three-dimensional spoiled gradient recalled echo sequences. Australas Radiol. 2004; 48:237–239.

64. Rangel-Guerra RA, Herrera J, Elizondo G, Gonzalez-Morantes J. Neurocysticercosis. Arch Neurol. 1988; 45:492.

65. Schantz PM, Tsang VC, Maddison SE. Serodiagnosis of neurocysticercosis. Rev Infect Dis. 1988; 10:1231–1233.

66. Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis. 1989; 159:50–59.

67. Wilson M, Bryan RT, Fried JA, Ware DA, Schantz PM, Pilcher JB, et al. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis. 1991; 164:1007–1009.

68. Rosas N, Sotelo J, Nieto D. ELISA in the diagnosis of neurocysticercosis. Arch Neurol. 1986; 43:353–356.

69. Nguekam , Zoli AP, Ongolo-Zogo P, Dorny P, Brandt J, Geerts S. Follow-up of neurocysticercosis patients after treatment using an antigen detection ELISA. Parasite. 2003; 10:65–68.

70. Garcia HH, Parkhouse RM, Gilman RH, Montenegro T, Bernal T, Martinez SM, et al. Serum antigen detection in the diagnosis, treatment, and follow-up of neurocysticercosis patients. Trans R Soc Trop Med Hyg. 2000; 94:673–676.

71. Correa D, Sandoval MA, Harrison LJ, Parkhouse RM, Plancarte A, Meza-Lucas A, et al. Human neurocysticercosis: comparison of enzyme immunoassay capture techniques based on monoclonal and polyclonal antibodies for the detection of parasite products in cerebrospinal fluid. Trans R Soc Trop Med Hyg. 1989; 83:814–816.

72. Harrison LJ, Joshua GW, Wright SH, Parkhouse RM. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol. 1989; 11:351–370.

73. Garcia HH, Harrison LJ, Parkhouse RM, Montenegro T, Martinez SM, Tsang VC, et al. A specific antigen-detection ELISA for the diagnosis of human neurocysticercosis. The Cysticercosis Working Group in Peru. Trans R Soc Trop Med Hyg. 1998; 92:411–414.

74. Fleury A, Hernández M, Avila M, Cárdenas G, Bobes RJ, Huerta M, et al. Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. J Neurol Neurosurg Psychiatry. 2007; 78:970–974.

75. Singh G. Neurocysticercosos in South-Central America and the Indian subcontinent. A comparative evaluation. Arq Neuropsiquiatr. 1997; 55:349–356.

76. Carpio A, Santillán F, León P, Flores C, Hauser WA. Is the course of neurocysticercosis modified by treatment with antihelminthic agents? Arch Intern Med. 1995; 155:1982–1988.

78. Salinas R, Prasad K. Drugs for treating neurocysticercosis (tapeworm infection of the brain). Cochrane Database Syst Rev. 2000; CD000215.

79. Sotelo J, del Brutto OH, Penagos P, Escobedo F, Torres B, Rodriguez-Carbajal J, et al. Comparison of therapeutic regimen of anticysticercal drugs for parenchymal brain cysticercosis. J Neurol. 1990; 237:69–72.

81. Proaño JV, Madrazo I, García L, García-Torres E, Correa D. Albendazole and praziquantel treatment in neurocysticercosis of the fourth ventricle. J Neurosurg. 1997; 87:29–33.

82. Göngora-Rivera F, Soto-Hernández JL, González Esquivel D, Cook HJ, Márquez-Caraveo C, Hernández Dávila R, et al. Albendazole trial at 15 or 30 mg/kg/day for subarachnoid and intraventricular cysticercosis. Neurology. 2006; 66:436–438.

83. Proaño JV, Madrazo I, Avelar F, López-Félix B, Díaz G, Grijalva I. Medical treatment for neurocysticercosis characterized by giant subarachnoid cysts. N Engl J Med. 2001; 345:879–885.

84. Del Brutto OH. Albendazole therapy for subarachnoid cysticerci: clinical and neuroimaging analysis of 17 patients. J Neurol Neurosurg Psychiatry. 1997; 62:659–661.

85. Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis. 2007; 44:549–553.

86. Colli BO, Martelli N, Assirati JA Jr, Machado HR, de Vergueiro Forjaz S. Results of surgical treatment of neurocysticercosis in 69 cases. J Neurosurg. 1986; 65:309–315.

87. Kelley R, Duong DH, Locke GE. Characteristics of ventricular shunt malfunctions among patients with neurocysticercosis. Neurosurgery. 2002; 50:757–761. discussion 761-762

88. Sharma BS, Gupta SK, Khosla VK. Neurocysticercosis: surgical considerations. Neurol India. 1998; 46:177–182.
89. Husain M, Jha D, Vatsal DK, Thaman D, Gupta A, Husain N, et al. Neuro-endoscopic surgery--experience and outcome analysis of 102 consecutive procedures in a busy neurosurgical centre of India. Acta Neurochir (Wien). 2003; 145:369–375. discussion 375-376
90. Husain M, Rastogi M, Jha DK, Husain N, Gupta RK. Endoscopic transaqueductal removal of fourth ventricular neurocysticercosis with an angiographic catheter. Neurosurgery. 2007; 60:4 Suppl 2. 249–253. discussion 254

91. Anandh B, Mohanty A, Sampath S, Praharaj SS, Kolluri S. Endoscopic approach to intraventricular cysticercal lesions. Minim Invasive Neurosurg. 2001; 44:194–196.

92. Cudlip SA, Wilkins PR, Marsh HT. Endoscopic removal of a third ventricular cysticercal cyst. Br J Neurosurg. 1998; 12:452–454.

93. Madrazo I, García-Rentería JA, Sandoval M, López Vega FJ. Intraventricular cysticercosis. Neurosurgery. 1983; 12:148–152.

94. Neal JH. An endoscopic approach to cysticercosis cysts of the posterior third ventricle. Neurosurgery. 1995; 36:1040–1043.

95. Bergsneider M. Endoscopic removal of cysticercal cysts within the fourth ventricle. Technical note. J Neurosurg. 1999; 91:340–345.
96. Bergsneider M, Holly LT, Lee JH, King WA, Frazee JG. Endoscopic management of cysticercal cysts within the lateral and third ventricles. J Neurosurg. 2000; 92:14–23.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download