Abstract
Tourette syndrome is a childhood-onset disorder characterized by a combination of motor and vocal tics, often associated with psychiatric comorbidities including attention deficit and hyperactivity disorder and obsessive-compulsive disorder. Despite an onset early in life, half of patients may present symptoms in adulthood, with variable degrees of severity. In select cases, the syndrome may lead to significant physical and social impairment, and a worrisome risk for self injury. Evolving research has provided evidence supporting the idea that the pathophysiology of Tourette syndrome is directly related to a disrupted circuit involving the cortex and subcortical structures, including the basal ganglia, nucleus accumbens, and the amygdala. There has also been a notion that a dysfunctional group of neurons in the putamen contributes to an abnormal facilitation of competing motor responses in basal ganglia structures ultimately underpinning the generation of tics. Surgical therapies for Tourette syndrome have been reserved for a small group of patients not responding to behavioral and pharmacological therapies, and these therapies have been directed at modulating the underlying pathophysiology. Lesion therapy as well as deep brain stimulation has been observed to suppress tics in at least some of these cases. In this article, we will review the clinical aspects of Tourette syndrome, as well as the evolution of surgical approaches and we will discuss the evidence and clinical responses to deep brain stimulation in various brain targets. We will also discuss ongoing research and future directions as well as approaches for open, scheduled and closed loop feedback-driven electrical stimulation for the treatment of Tourette syndrome.
Tourette syndrome (TS) is a childhood-onset neuropsychiatric disorder which encompasses a spectrum of symptoms inclusive of motor and phonic tics, which may be accompanied by neuropsychiatric comorbidities such as attention-deficit/hyperactivity disorder (ADHD), obsessive compulsive disorder (OCD), impulse control disorder, and other behavioral manifestations.1 According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V) and the International Classification of Diseases, tenth edition criteria, the patients must have 1) a combination of chronic motor and phonic tics, and 2) these must occur several times a day for at least 1 year. 3) The symptoms must have an onset before the age of 18 years and 4) these symptoms must not be explained by other medical/neurological conditions. Alternatively the Tourette Syndrome Classification Group2 published a set of diagnostic criteria with slight differences as compared to the international classifications, with the age of onset prior to 21. The Tourette Syndrome Classification Group embraced the idea that the anatomic location, number, frequency, complexity, type, and severity of the tics could change over time. The latter classification also included the notion that the tics must be witnessed by a reliable examiner, either directly or by videotaping.2 In 2010 the National Institutes for Mental Health proposed a new paradigm for classification of psychiatric disorders, the Research Domain Criteria (RDoC). In contrast to the ICD and DSM, the RDoC identifies constructs and subconstructs based on genetic, structural, and connectivity factors believed to underlie similar behavioral and physiological responses.3,4 TS in this classification fell under the construct of habits in the positive valence systems domain, which is characterized by repetitive, stereotypic, and compulsive behaviors. These behaviors have been thought to be mediated by dopamine and other neurochemical dysfunction in circuits involving the striatum and prefrontal cortex.
The TS motor and phonic tic course typically wanes in most patients as they transition from adolescence into adulthood, however symptoms persist in about half of patients into the adult years.1 Despite several pharmacological approaches, a number of patients remain refractory to therapy and have a persistent and impaired quality of life. There may also be disabling social embarrassment due to both the tics and to psychiatric comorbidities. Severe medication-refractory TS cases have been addressed by surgical therapies since the 1960s. The early therapies focused on ablation of motor and also limbic targets.5 In 1999, Vandewalle et al.6 reported the first case of a patient with TS who was implanted with a deep brain stimulation (DBS) device. The intervention involved bilateral electrode implantation in the centromedian nucleus of the thalamus. At 4 months, the authors reported a significant reduction from 38 to 8 tics per minute (79% improvement) during active stimulation.
The use of TS DBS has generated several case reports and series with stimulation not only of centromedian (CM), but also now inclusive of other targets believed to be part of a dysfunctional neural network ultimately contributing to the underlying pathophysiology thought to be responsible for TS. There has been reported large variability in response to DBS, and large well-powered prospective trials have yet to be conducted. The Tourette Syndrome Association has commissioned a large international database of all implants worldwide.
The pathophysiology of TS remains unknown. Experts have hypothesized the existence of a group of hyperactive dopaminergic neurons that may contribute to a dysfunctional cortico-striato-thalamo-cortical circuit and ultimately lead to decreased cortical inhibition. It has also been pointed out by many experts that this hypothesis cannot explain all of the manifestations of TS.7,8 Additionally, there has been recent data revealing structural changes in the globus pallidus, reduced cyclic adenosine monophosphatea in cortex and striatum, and an increase in dopamine uptake in striatum.1 These largely pathological findings support the notion of enhanced dopaminergic activity. It is also postulated that a developmental defect in migration of gamma-aminobutyric acid (GABA) neurons could play a role in the overall the imbalance of the cortico-striato-thalamic circuit.1,9 The dopaminergic hypothesis has been attractive to many scientists, particularly since dopaminergic blocking drugs result in tic suppression for many TS subjects. Dopamine, however, is likely only one part of the underlying pathophysiological story underpinning TS.
There is growing evidence to support the hypothesis that cortical excitability is abnormally increased in TS. Studies utilizing functional magnetic resonance imaging8,10 and transcranial magnetic stimulation1,7 have shown an increase in excitation of M1 cortical areas that seemed to correlate with the severity of TS.7 Also there appears to be an increase in activation of primary sensorimotor and secondary motor cortices during execution of motor tasks (such as finger tapping),8 as well as coupling of the supplementary motor area and the contralateral M1 motor cortical areas during the movement programming phase (planning to execute a movement) suggesting that this could be an adaptive mechanism that it may also be a marker for TS.8
The execution of motor tasks is known to occur through the activation of a complex circuit involving interaction between the cortex and the basal ganglia. The striatum receives excitatory glutamatergic input from the motor cortex and projects to other basal ganglia structures including the internal portion of the globus pallidus (GPi) and the substantia nigra pars reticulata. These two structures are the main outputs to thalamic nuclei and they function largely through GABAergic projections.11 Non-human primate experiments have revealed that microstimulation of discrete striatal areas can result in stereotyped movements of the extremities.11 It has been suggested that in TS patients, a population of striatal neurons becomes abnormally active, leading to excessive inhibition of basal ganglia output to the thalamus which generates competing motor patterns that could ultimately be the source of tics.11 This network hypothesis, though compelling, may only be part of the story as other factors are likely important to the genesis of tics and complex behavioral manifestations.
Another compelling hypothesis of TS pathogenesis involves dysfunction of basal ganglia dopaminergic structures and the connections to frontocortical circuits.11 Mink11 has proposed a schema where, in a normal person, there is tonically active inhibitory output of the basal ganglia in connections to the cortex and brainstem, suppressing motor pattern generators (MPGs). With the initiation of a purposeful movement, the basal ganglia projections increase their firing rate towards competing MPGs, suppressing undesired motor patterns. During a tic, it is postulated that tics arise from a focal population of striatal neurons that become abnormally hyperactive and result in downstream inhibition into the basal ganglia structures, which in turn may reduce their inhibitory output into cortex and brainstem. Therefore unwanted intrusive MPGs may possibly be translated into repetitive abnormal stereotyped movements.11
The amygdala is involved in the processing of a variety of stimuli inclusive of facial emotional expression, sounds necessary for communication, and also for processing of music.12 The amygdala is also thought to mediate many manifestations of neuro-psychiatric diseases such as depression, anxiety, and antisocial personality disorders.12 The subnuclei of the amygdala can be structurally subdivided into laterobasal, centromedial and superficial groups each associated with specific functional properties as evidenced by recent neuroimaging studies.12
Projections from the amygdala (in particular the superficial nuclei group) not only are important for learning, behavior and emotional distress, but are also thought to play a role in tic suppression in TS patients through close interactions with the frontal cortices.13 Peterson et al.14 observed larger amygdala volumes in TS children when compared to age-matched controls, but smaller amygdala volumes in adult TS patients. These findings suggest that there is a neuromodulatory mechanism involving the amygdala circuits that may be lost in TS patients whose tics persisted into adulthood.13
Neurosurgical interventions for TS have been around since 1955. Early in the history of TS surgery, frontal lobotomies and leucotomies were conducted for cases that were refractory to pharmacological and other therapies.15 There has, since the early Hassler thalamotomy cases, been specific targeting of basal ganglia structures and thalamic nuclei. Early approaches utilized stereotactic coagulation of thalamic nuclei including the intralaminar and centromedian thalamic, as well as the internal portion of ventralis oralis anterior (VOA). Other targets such as GPi, and even isolated reports of globus palidus externa (GPe)5 and subthalamic nucleus,16 have been emerging as potentially successful. The nucleus accumbens and anterior limb of the internal capsule region, a target used successfully for treatment of obsessive compulsive disorder, a common comorbidity in TS, have been used in a handful of patients perhaps with less robust response. In 1999 Vandewalle et al.6 reported the results of the first 3 cases of TS patients who underwent thalamic DBS. These initial patients were implanted to address symptoms unresponsive to medical and behavioral therapies. Since this early report, others have attempted different targets and approaches. A summary of the proposed DBS targets is provided in Fig. 1.
In 1970, Hassler and Dieckmann published a case series including 3 patients who underwent stereotactic coagulation of thalamic nuclei for treatment of severe TS.17 The authors targeted the CM, rostral intralaminar, and the internal portion of VOA nuclei of the thalamus. All 3 patients obtained positive responses, and the original paper cites that these three individuals had improvement in motor obsessions (100%), involuntary shouting (90%), and coprolalia (70%). The authors argued that one patient with less improvement in coprolalia was the one who did not receive supplementary coagulation in the VOA interna, an area that projects to the facial region of the motor cortex.17
In 1999, Vandewalle et al.6 described the first cases of intractable TS addressed with DBS therapy. Her group used the same coordinates as Hassler and Dieckman's lesions.6 Preoperatively, the index patient had on average 38 tics per minute, and, at 4 months, this number improved to 8 tics per minute. Only repetitive eye blinking after the bilateral stimulators were turned on was documented. The authors stimulated at a voltage of 4 volts (V), a frequency of 130 hertz (Hz) and a pulse width of 450 microseconds (µs). Follow-up at 1 year postoperatively revealed the voltage was 1.5 V and this level was sufficient to abolish tics. The authors also comment on the importance of including stimulation of the VOA region due to its connections with the facial parts of the premotor cortex areas, and its hypothesized importance to suppression of motor and vocal tics.6
The thalamus is a major relay sending excitatory information back to the cortex. The centromedian-parafascicular (CM-pf) complex in particular, has been thought to be altered by DBS through decreased dopaminergic input while on stimulation,18 which is consistent with the theory proposed by Mink.11 The internal portion of VOA, due to the connections with facial areas in the motor cortex was previously targeted for additional coagulations by Hassler and Dieckman to address recurrent facial and vocal tics. This extra intervention led to improvement of symptoms.6,15
In 2008, Servello et al.19 published an open label study of 18 patients who underwent bilateral thalamic DBS and had improvement in the Yale Global Tic Severity Scale (YGTSS). There was a mean change in tic score of 80.8 prior to surgery to 28.6 after surgery (65% mean improvement), with a subgroup of patients experiencing progressively steady improvement, and another subgroup requiring more adjustments to the DBS. Ackermans et al.20 enrolled 8 patients with bilateral thalamic DBS and randomized them to the devices turned on immediately following surgery versus delayed activation at a three month timepoint. The results revealed a marked improvement in the YGTSS with the device on.
To address the question of whether unilateral or bilateral stimulation was required, Maciunas et al.21 randomized 5 patients on a weekly basis to unilateral (left or right), bilateral, or to no stimulation for the first 28 days following the surgery. Unilateral stimulation provided some benefit, however patients who received bilateral stimulation had a higher rate improvement in the raw tic counts (53%) in the patients randomized to the on-on state.
Okun et al.22 randomized 5 patients to initiate stimulation at postoperative day 30 or 60, and also patients were evaluated with stimulation "off", "on" continuously or "on" scheduled. At 6 month follow-up, there was a 38% reduction in the total Modified Rush Tic Rating Scale (from 16.2±2.3 to 10.4±4.8), as well as a 58% improvement in the phonic tic severity (from 3.8±0.4 to 1.6±1.8), and a 19% reduction in the total YGTSS from 91.6±8.8 to 73.8±11.5 as well as in the subscores for motor severity and impairment. Both continuous and scheduled stimulation showed benefit and although scheduled stimulation did not achieve the expected goal of 50% reduction in the number of tics, this was the first study to demonstrate the concept that scheduled stimulation may be effective for symptomatic tic control.
The Table 1 summarizes the main outcomes from studies targeting thalamic nuclei.
The globus pallidus is an important structure in the pathophysiology of TS, as it is the structure downstream from the striatum where diseased cell groups are postulated to be responsible for initiating stereotyped and repetitive movements.11 DBS approaches to both GPi and GPe5 have been attempted, and both have had reported successes. Interestingly, the globus pallidus has been shown to correlate with contralateral symptoms in a recent case report of tics improving with GPi stimulation.23
Two different approaches to GPi DBS have been attempted; anteromedial (amGPi) and posteroventrolateral (pvlGPi). To date there have been similar rates of success. In 2005, Diederich et al.24 reported the first case of a TS patient who underwent bilateral pvlGPi. At 14 months the number of tics per minute had improved by 66%, and the total YGTSS score improved from 83 to 44 (47% improvement). The patient appeared to experience a sustained effect of brain stimulation with the subjective urge to tic not returning for 48 hours after the brain stimulator was left off. Larger series have also followed GPi patients. In an open label study performed by Cannon et al.25 that enrolled 11 TS patients with bilateral GPi stimulation, six patients had a reduction in the number of tics greater than 50%, and this was demonstrated across multiple tic severity scales. Four patients had marked improvement, but did not reach the goal of 50% improvement. Only one patient failed to have marked improvement as measured by the YGTSS, and this was attributed by the authors to a severe and disabling presentation of TS.
Unilateral stimulation of pvlGPi has been reported for two patients,26 with greater than 50% reduction on the YGTSS. This improvement was sustained throughout a 12-month follow up period. Both patients experienced recurrence of tics 24 hours after the DBS was turned off, and a return to baseline improvement after the stimulator was turned back on.
Vilela Filho et al. hypothesized that TS was the clinical expression of a hyperactive GPe and prefrontal area.5 Based on experience from previous stereotatic proceduress in GPe, Piedimonte et al.5 reported 1 case of a patient with intractable TS who underwent bilateral GPe DBS. On chronic stimulation, the patient had benefits within 24 hours, and reached a plateau 10 days after the stimulators were turned on. The tics returned about 2 hours after the stimulators were turned off, and improved again 1 hour after the stimulators were reactivated. The patient was able to discontinue medication and remained stable for two years after the surgery.
Table 2 summarizes the studies and case reports of GPi DBS in TS patients.
There have been conflicting therapeutic responses and more modest results reported in anterior limb of internal capsule (AIC)/nucleus accumbens (NA) DBS. A few case reports have evaluated the response of DBS to this region that is commonly used in obsessive compulsive disorder; a common comorbidity in TS.
Kuhn et al.27 described a patient with TS with severe OCD as a comorbid condition. This patient had DBS leads implanted bilaterally into the AIC-NA. The patient experienced marked improvement of OCD symptoms and a 40-50% improvement of tics, suggesting that the NA could be involved in the complex circuitry of TS. Flaherty et al.28 reported a case of 37-year-old patient with severe TS consisting of motor and vocal tics, without OCD or depression, who underwent DBS surgery targeting the AIC. At 18 month follow up, the patient had experienced a 25% decrement in the YGTSS and a 45% reduction in tic frequency and severity according to patient logs. Neuner et al.29 reported a case of severe TS, OCD and self-injurious behavior who after bilateral AIC-NA DBS at 36 months experienced a 44% improvement. Burdick et al.30 however, reported a case of a patient with severe and disabling OCD who also had mild TS. This patient received bilateral AIC-NA DBS leads, and at 30 months follow up did not experience a changes in tics.
Over the years there has been an increasing knowledge of the pathophysiology of TS and other diseases due to the use of single cell and local field potential (LFP) recordings pre- or post-operatively. There has been a notion that pathological frequencies or oscillations may contribute to the occurrence of symptoms. While excessive beta oscillations (11-30 Hz) have been discovered in hypokinetic conditions such as Parkinson's disease,31 low frequency oscillations (2-7 Hz) may also be encountered in hyperkinetic conditions such as dyskinesias and dystonias.31 There is very little overall known on this topic, however the field is evolving quickly.
In anesthetized TS patients (without ongoing tics), microelectrode recordings from thalamic nuclei have revealed a pattern of low frequency firing in a burst pattern.31 While awake, these patients have LFPs characterized by alpha and low frequency activity (in the absence of beta band oscillations) which seem to correlate with the clinical phenotype of TS, as patients with less tics and more OCD features seem to have fewer low frequency oscillations than patients in whom tics were a predominant feature.31 Similar to thalamus, GPi has shown low frequency oscillations that seem to precede the electromyographic recording of the tic by 50 ms or more, suggesting that in the GPi this activity could reflect the sensations that are premonitory to a motor tic,31 however this remains speculative. These specific frequency oscillations, in particular in thalamus have been targeted and shown to be dramatically altered by application of DBS.32
Anterior limb of internal capsule and NA are structures known to play an important role in the pathophysiology of the reward system and seem to play an important role in patients with OCD, which is a typical comorbidity in TS. These structures have also been used for LFP recordings, and researchers have observed high beta power oscillations in the NA in comparison to VOA/CM-pf thalamus. This may be a physiological marker of the OCD activity that would lead to the lower frequency oscillations found in the thalamic nuclei.31
As research progresses, more efficient therapies may possibly deliver stimulation instead of in a continuous or scheduled fashion, in a more responsive paradigm that triggers off of pathological oscillations.
New stimulation techniques are being developed and some have moved into clinical trials. DBS as a therapeutic technique for movement disorders and neuropsychiatric disorders has been previously delivered in an open-loop paradigm. These paradigms focus on a pre-programmed chronic and continuous stimulation pattern,33 regardless of the internal state of the system, or the environmental factors. TS however, presents in a paroxysmal pattern, characterized by intermittent episodes of motor and/or vocal tics in addition to neuropsychiatric components.1 This presentation in many ways is similar to epilepsy. Newer stimulation delivery devices, can be personalized to the frequency and duration of a behavioral manifestation. These devices, in which stimulation is independent of functional neural feedback have been initially designed to treat seizures (open loop approaches). In this scenario they act to inhibit seizure propagation and to alter seizure thresholds and have been shown to be safe and efficacious.34 The only TS study to date using scheduled rather than constant stimulation, reported clinical outcomes of 5 patients after 6 months using a novel approach.22 Targeting the centromedian brain region, safety and feasibility of intermittent stimulation has been established, however outcomes were less than expected.
Responsive or adaptive DBS (aDBS), so-called closed-loop stimulation devices, rely on functional neural feedback, such as abnormal electrographic discharges or more recently on neurochemical feedback. These closed loop approaches may adjust stimulation parameters and shorten "after-discharges" elicited during functional mapping.34,35 Currently, DBS research has been focusing on how to interpret brain activity and use it as feedback to control delivery of therapeutic stimulation. There has been an emerging development of responsive stimulation in the treatment of Parkinson's disease and a growing discussion about proposing cortical stimulation rather than DBS.36 Recent proof-of-principle studies have shown that by personalizing and optimizing stimulation in real time, efficacy and efficiency of continuous DBS could be improved. LFPs have been reported to provide the most relevant biomarker to close the loop.37 Brain-computer interface (BCI) systems have been tested to control the timing of when stimulation is delivered.38 These BCI systems use the beta activity in the LFP recorded directly from the electrode in order to regulate stimulation. In 8 patients with advanced PD, aDBS was demonstrated to be 30% more effective than conventional DBS therapy while delivering <50% of the stimulation of current DBS strategies. This study suggests that it is possible to track an LFP biomarker from the site of stimulation and that aDBS could be more efficient and efficacious than conventional neuromodulation for PD. Another potential alternative feedback signal is the electrically evoked compound action potential (ECAP). This signal results from activation of an ensemble of neural elements following each DBS pulse. A novel instrumentation that uses commercial amplifiers in a custom serial configuration to suppress the artifact and record short latency ECAPs after each DBS pulse was developed and demonstrated that ECAPs can be recorded with high fidelity during DBS therapy. This novel instrumentation was validated through both in vitro and in vivo testing.39 Recent animal studies have shown that stimulation-induced changes in neurotransmitter release can be associated with the therapeutic benefit of DBS. In a rodent model of DBS, mathematical models were used to describe relationships between stimulation-evoked extracellular dopamine response and DBS parameters and have shown that adjusting stimulation intensity can modulate dopamine concentration.40 Open- and closed-loop configurations for DBS therapy are demonstrated in Fig. 2.
Deep brain stimulation has been shown to be a promising therapy for TS, more so for motor and phonic tics than for associated comorbidities, such as OCD, ADHD, and self-injurious behavior. Overall, a better understanding of the circuitry involved in TS and the mechanisms of brain stimulation will speed development of new techniques and devices. Deciding the best scales and ways to measure outcome will impact the future development of the TS DBS field. Up to 9 targets have been explored individually or in combination, for the treatment of TS with reported improvement in tics. New targets are currently being investigated to treat symptoms less responsive to standard DBS.41
Technological advances in DBS devices or in the systems of stimulation delivery, may enhance clinical outcomes.42 Directional steering through segmented electrodes capable of modeling the electrical field to better target a desired structure or pathway with less side effects, such as a novel DBS electrode with 32 contacts has been shown to be safe, well tolerated, to decrease the thresholds for side effects while improving the therapeutic window of DBS.43 Local field potentials can be also used to close the loop and to identify information regarding high-level sensory processing, perception, and locomotor activity. Whole-brain electropysiological brain activity is measured using far-field sensors located on the scalp by electroencephalogram or directly on the brain surface by electrocorticography (ECoG). A system that combines activity analysis within cortical (ECoG) and subcortical (LFP) networks will likely provide a better depiction of network dynamics.7
Our group at the University of Florida Center for Movement Disorders and Neurorestoration is currently conducting a research study on TS neural network and combining bilateral thalamic stimulation with cortical ECoG strips implanted on the primary and premotor cortices. The goal is to investigate spectral features of tics compared to baseline and volitional movements, and also to investigate the role of phase amplitude coupling in the cortex. Preliminary results have shown that tics are spectrally obvious and can be detected, and strong coupling after application of therapeutic settings can be observed (unpublished data). Further analysis will uncover a more complete understanding on the electrophysiology of tics and how DBS is effective in treating this disorder.
In addition, the development of new neuromodulation techniques such as optogenetics or thermogenetics, may help to better rationalize therapy and to redesign brain stimulation and to make it more efficient. Optogenetics refers to the integration of optics and genetics to achieve gain- or loss-of-function of well-defined events within specific cells of living tissue,44,45,46 by using light to control activity of neurons which have been modified to express light-sensitive proteins, such as channelrhodopsin. Thermogenetics uses temperature to drive neural activity.47 To date, two thermosensitive Transient Receptor Potential (TRP)-based tools have been developed for use. Their thermal sensitivity is such that a neuron expressing a thermoTRP can switch from silent to robustly active in response to temperature shifts as small as 1-2℃. These novel approaches have been widely employed in basic science research. However, with the continual improvements to the molecular toolbox of genetically encoded neuronal proteins, new technologies may support new therapeutic modalities that can be ultimately applied in humans. In the near future, we will likely employ multiple leads to uncover the neural networks and to monitor physiology in real-time in an awake behaving human.
Figures and Tables
 | Fig. 1Summary of the proposed targets for DBS in Tourette syndrome. A: The thalamus in a coronal view, wherein the centromedian-parafascicular (CM-pf) complex is targeted. B: A cross-sectional view of the thalamus in detail, demonstrating the anatomical relation of the CM nucleus with the anterior portion of the ventralis oralis (VOA) nucleus, targeted by additional coagulations during the initial thalamotomy studies. C: Different areas of globus pallidus interna (GPi) that have been targeted, and D: A less studied, although with some reports of satisfactory clinical response, the anterior limb of internal capsule (AIC) and nucleus accumbens (NA). DBS: deep brain stimulation, GPe: globus palidus externa, SN: substantia nigra pars reticulata, STN: subthalamic nucleus, VOP: posterior portion of the ventralis oralis. |
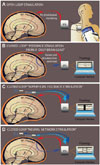 | Fig. 2Summary of the proposed approaches for DBS in Tourette syndrome. A: The conventional stimulation in an open loop fashion currently used widely in movement disorders, where energy is continuously delivered to a target, with parameters set by a clinician. B: The concept of closed loop DBS, where energy is delivered as a real time feedback response to physiological changes detected by LFPs through a computer interface. C: An alternative mode of closed loop DBS, in which energy is delivered as a real time feedback to changes in the surface of the brain, detected by EEG and/or ECoG. D: The novel concept of neural network stimulation, where the stimulation is delivered in a feedback response to a physiological changes detected at the cortical level through EEG and ECoG and subcortical level detected by LFPs, yielding delivery of electrical stimulation through both the DBS and ECoG leads. DBS: deep brain stimulation, ECoG: electrocorticography, EEG: electroencephalogram, LFP: local field potential. |
Table 1
Summary of case reports and series for thalamic deep brain stimulation

| Authors | Number of patients | Study characteristics | Follow-up | Outcomes |
|---|---|---|---|---|
| Vandewalle et al.6 | 1 patient | Target: bilateral CM-substantial periventricularis-VOi | 1 year | Suppression of tics, with exception of repetitive eye blinking |
| Visser-Vandewalle et al.48 | 3 patients | Target: bilateral CM-substantial periventricularis-VOi | 8 months to 5 years | Marked improvement of motor and vocal tics in all 3 patients |
| Houeto et al.49/Welter et al.50 | 3 patients | Study comparing bilateral CM-pf thalamic DBS versus bilateral GPi stimulation | 20-60 months | Significant improvement of YGTSS scores on either thalamic or GPi stimulation in comparison to sham stimulation. No added benefit of combined stimulation of both targets |
| Maciunas et al.21 | 5 patients | Target: anterior portion of the CM-pf complex. Patients were randomized into unilateral, bilateral or no electrode activation during the first 28 days | 1 year | There was a 44% reduction in the mean YGTSS, corresponding to 3 patients whose improvement was detected by the scales and video assessment. Two patients experienced recurrence of tics during the open label phase of programming |
| Bajwa et al.51 | 1 patient | Case report of 1 patient with bilateral thalamic DBS, unclear of what nucleus was being targeted | 2 years | 66% improvement of YGTSS at 24 months |
| Servello et al.19/Porta et al.52 | Initially 18 patients at 1 year follow up, 15 at 2 years follow up | Bilateral CM-pf+VOA. Initial 1 year follow up reported by Servello et al. 200819 | 2 years | Statistically significant reduction in the tic severity by YGTSS, improvement of OCD symptoms, anxiety, depression, and quality of life |
| Shields et al.53 | 1 patient | Patient initially with bilateral DBS leads in anterior limb of internal capsule, with poor response, resulting in permanent lead damage due to retrocolic jerks. Leads were replaced by bilateral leads targeting CM-pf | 21 months (18 months after AIC placement and 3 months after replacement by thalamic leads) | 42% decrease in total motor tic score, 40% in total phonic tic score, 41% in total tic score, 50% in overall impairment, and 46% in global severity using the YGTSS |
| Vernaleken et al.18 | 1 patient | Target: bilateral pf-dorsomedial thalamus | 6 months | Patient initially failed bilateral GPi stimulation, but was severely disabled by the tics, so he had implanted bilateral thalamic DBS leads, with significant response on the YGTSS |
| Ackermans et al.54 | 2 patients | Bilateral CM-pf+VOA | 6 and 10 years | Case 1 had 90.1% tic reduction at 5 years, sustained at 10 years (92.6%). Case 2 had 82% tic reduction at 8 months, with slight worsening at 6-year follow up (78%) |
| Kaido et al.55 | 3 patients | Target: bilateral CM-pf+VOA | 12 months | Steady improvement in the YGTSS and social impairment scores |
| Ackermans et al.20 | 6 patients | Bilateral CM-pf+VOA. Patients randomized into off or on state of the DBS | 1 year | Substantial tic reduction by the YGTSS when comparing on and off states |
| Lee et al.56 | 1 patient | 1 patient, bilateral CM-pf DBS | 18 months | 81% improvement in total tics, 58% improvement in YGTSS |
| Savica et al.57 | 3 patients | 3 patients, CM-pf nucleus | 1 year | YGTSS motor subscore improved 45-80% and impairment subscore improved 75-80% |
| Okun et al.22 | 5 patients | Target: bilateral CM thalamus. Patients evaluated on continuous and intermittent stimulation | 6 months | Statistically significant improvement in the YGTSS, MRTSS, and phonic tic severity score on both continuous and intermittent modes |
Table 2
Summary of case reports and series involving globus palidus interna DBS

| Authors | Number of patients | Study characteristics | Follow-up | Outcomes |
|---|---|---|---|---|
| Diederich et al.24 | 1 patient | Target: bilateral posteroventro-lateral GPi | 14 months | Tic reduction of 66% |
| Ackermans et al.58 | 1 patient | 1 patient with bilateral CM-substantial periventricularis-VOi and bilateral GPi | 1 year | Patient improved from 28 to 2 tics/min. Authors stimulated different targets separately during the postoperative period, and GPi stimulation had a greater tic reduction, so the GPi electrodes were connected to the pulse generators |
| Gallagher et al.23 | 1 patient | Target: bilateral GPi | Not available | Patient had marked improvement of her tics. Unfortunately she required removal of left lead due to infection, and tics reappeared on the right side of her face and arm |
| Shahed et al.59 | 1 patient | Target: bilateral GPi | 6 months | 84% improvement in the YGTSS |
| Dehning et al.60 | 1 patient | Target: bilateral GPi | 1 year | Complete resolution of motor and vocal tics at 12 months from surgery |
| Dueck et al.61 | 1 patient | Target: bilateral GPi | 1 year | No significant improvement in YGTSS or use of antipsychotics |
| Cannon et al.25 | 11 patients | Target: bilateral GPi at the caudal border. Open label study | 3 months | 91% of the patients reported improvement. Six patients achieved the goal of clinical response, defined by reduction on YGTSS greater than 50% |
| Dong et al.26 | 2 patients | Target: unilateral GPi | 1 year | Greater than 50% reduction in the YGTSS in both patients |
| Massano et al.62 | 1 patient | Target: bilateral anteromedial GPi | 2 years | 60.5% reduction in the YGTSS |
References
2. The Tourette Syndrome Classification Study Group. Definitions and classification of tic disorders. Arch Neurol. 1993; 50:1013–1016.
3. Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010; 167:748–751.

4. Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012; 14:29–37.

5. Piedimonte F, Andreani JC, Piedimonte L, Graff P, Bacaro V, Micheli F, et al. Behavioral and motor improvement after deep brain stimulation of the globus pallidus externus in a case of Tourette's syndrome. Neuromodulation. 2013; 16:55–58. discussion 58.

6. Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999; 353:724.

7. Gilbert DL, Bansal AS, Sethuraman G, Sallee FR, Zhang J, Lipps T, et al. Association of cortical disinhibition with tic, ADHD, and OCD severity in Tourette syndrome. Mov Disord. 2004; 19:416–425.

8. Franzkowiak S, Pollok B, Biermann-Ruben K, Südmeyer M, Paszek J, Thomalla G, et al. Motor-cortical interaction in Gilles de la Tourette syndrome. PLoS One. 2012; 7:e27850.

9. Singer HS, Morris C, Grados M. Glutamatergic modulatory therapy for Tourette syndrome. Med Hypotheses. 2010; 74:862–867.

10. Baym CL, Corbett BA, Wright SB, Bunge SA. Neural correlates of tic severity and cognitive control in children with Tourette syndrome. Brain. 2008; 131(Pt 1):165–179.

11. Mink JW. Basal ganglia dysfunction in Tourette's syndrome: a new hypothesis. Pediatr Neurol. 2001; 25:190–198.

12. Ball T, Rahm B, Eickhoff SB, Schulze-Bonhage A, Speck O, Mutschler I. Response properties of human amygdala subregions: evidence based on functional MRI combined with probabilistic anatomical maps. PLoS One. 2007; 2:e307.

13. Werner CJ, Stöcker T, Kellermann T, Wegener HP, Schneider F, Shah NJ, et al. Altered amygdala functional connectivity in adult Tourette's syndrome. Eur Arch Psychiatry Clin Neurosci. 2010; 260:Suppl 2. S95–S99.

14. Peterson BS, Choi HA, Hao X, Amat JA, Zhu H, Whiteman R, et al. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Arch Gen Psychiatry. 2007; 64:1281–1291.

16. Martinez-Torres I, Hariz MI, Zrinzo L, Foltynie T, Limousin P. Improvement of tics after subthalamic nucleus deep brain stimulation. Neurology. 2009; 72:1787–1789.

17. Rickards H, Wood C, Cavanna AE. Hassler and Dieckmann's seminal paper on stereotactic thalamotomy for Gilles de la Tourette syndrome: translation and critical reappraisal. Mov Disord. 2008; 23:1966–1972.

18. Vernaleken I, Kuhn J, Lenartz D, Raptis M, Huff W, Janouschek H, et al. Bithalamical deep brain stimulation in tourette syndrome is associated with reduction in dopaminergic transmission. Biol Psychiatry. 2009; 66:e15–e17.

19. Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2008; 79:136–142.

20. Ackermans L, Duits A, van der Linden C, Tijssen M, Schruers K, Temel Y, et al. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain. 2011; 134(Pt 3):832–844.

21. Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007; 107:1004–1014.

22. Okun MS, Foote KD, Wu SS, Ward HE, Bowers D, Rodriguez RL, et al. A trial of scheduled deep brain stimulation for Tourette syndrome: moving away from continuous deep brain stimulation paradigms. JAMA Neurol. 2013; 70:85–94.

23. Gallagher CL, Garell PC, Montgomery EB Jr. Hemi tics and deep brain stimulation. Neurology. 2006; 66:E12.

24. Diederich NJ, Kalteis K, Stamenkovic M, Pieri V, Alesch F. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: a case report. Mov Disord. 2005; 20:1496–1499.

25. Cannon E, Silburn P, Coyne T, O'Maley K, Crawford JD, Sachdev PS. Deep brain stimulation of anteromedial globus pallidus interna for severe Tourette's syndrome. Am J Psychiatry. 2012; 169:860–866.

26. Dong S, Zhuang P, Zhang XH, Li JY, Li YJ. Unilateral deep brain stimulation of the right globus pallidus internus in patients with Tourette's syndrome: two cases with outcomes after 1 year and a brief review of the literature. J Int Med Res. 2012; 40:2021–2028.

27. Kuhn J, Lenartz D, Mai JK, Huff W, Lee SH, Koulousakis A, et al. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome. J Neurol. 2007; 254:963–965.

28. Flaherty AW, Williams ZM, Amirnovin R, Kasper E, Rauch SL, Cosgrove GR, et al. Deep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: technical case report. Neurosurgery. 2005; 57:4 Suppl. E403. discussion E403.

29. Neuner I, Halfter S, Wollenweber F, Podoll K, Schneider F. Nucleus accumbens deep brain stimulation did not prevent suicide attempt in tourette syndrome. Biol Psychiatry. 2010; 68:e19–e20.

30. Burdick A, Foote KD, Goodman W, Ward HE, Ricciuti N, Murphy T, et al. Lack of benefit of accumbens/capsular deep brain stimulation in a patient with both tics and obsessive-compulsive disorder. Neurocase. 2010; 16:321–330.

31. Priori A, Giannicola G, Rosa M, Marceglia S, Servello D, Sassi M, et al. Deep brain electrophysiological recordings provide clues to the pathophysiology of Tourette syndrome. Neurosci Biobehav Rev. 2013; 37:1063–1068.

32. Maling N, Hashemiyoon R, Foote KD, Okun MS, Sanchez JC. Increased thalamic gamma band activity correlates with symptom relief following deep brain stimulation in humans with Tourette's syndrome. PLoS One. 2012; 7:e44215.

33. Hariz M. Twenty-five years of deep brain stimulation: celebrations and apprehensions. Mov Disord. 2012; 27:930–933.

34. Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol. 2006; 19:164–168.

35. Chang SY, Kimble CJ, Kim I, Paek SB, Kressin KR, Boesche JB, et al. Development of the Mayo Investigational Neuromodulation Control System: toward a closed-loop electrochemical feedback system for deep brain stimulation. J Neurosurg. 2013; 119:1556–1565.

36. Beuter A, Lefaucheur JP, Modolo J. Closed-loop cortical neuromodulation in Parkinson's disease: an alternative to deep brain stimulation? Clin Neurophysiol. 2014; 125:874–885.

37. Bronte-Stewart H, Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN beta-band profile in Parkinson's disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol. 2009; 215:20–28.

38. Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013; 74:449–457.

39. Kent AR, Grill WM. Instrumentation to record evoked potentials for closed-loop control of deep brain stimulation. Conf Proc IEEE Eng Med Biol Soc. 2011; 2011:6777–6780.

40. Grahn PJ, Mallory GW, Khurram OU, Berry BM, Hachmann JT, Bieber AJ, et al. A neurochemical closed-loop controller for deep brain stimulation: toward individualized smart neuromodulation therapies. Front Neurosci. 2014; 8:169.

41. Malaty IA, Akbar U. Updates in medical and surgical therapies for Tourette syndrome. Curr Neurol Neurosci Rep. 2014; 14:458.

42. Marceglia S, Rossi L, Foffani G, Bianchi A, Cerutti S, Priori A. Basal ganglia local field potentials: applications in the development of new deep brain stimulation devices for movement disorders. Expert Rev Med Devices. 2007; 4:605–614.

43. Chaturvedi A, Foutz TJ, McIntyre CC. Current steering to activate targeted neural pathways during deep brain stimulation of the subthalamic region. Brain Stimul. 2012; 5:369–377.

45. LaLumiere RT. A new technique for controlling the brain: optogenetics and its potential for use in research and the clinic. Brain Stimul. 2011; 4:1–6.

46. Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011; 71:9–34.

47. Bernstein JG, Garrity PA, Boyden ES. Optogenetics and thermogenetics: technologies for controlling the activity of targeted cells within intact neural circuits. Curr Opin Neurobiol. 2012; 22:61–71.

48. Visser-Vandewalle V, Temel Y, Boon P, Vreeling F, Colle H, Hoogland G, et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg. 2003; 99:1094–1100.

49. Houeto JL, Karachi C, Mallet L, Pillon B, Yelnik J, Mesnage V, et al. Tourette's syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005; 76:992–995.

50. Welter ML, Mallet L, Houeto JL, Karachi C, Czernecki V, Cornu P, et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008; 65:952–957.

51. Bajwa RJ, de Lotbinière AJ, King RA, Jabbari B, Quatrano S, Kunze K, et al. Deep brain stimulation in Tourette's syndrome. Mov Disord. 2007; 22:1346–1350.

52. Porta M, Brambilla A, Cavanna AE, Servello D, Sassi M, Rickards H, et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009; 73:1375–1380.

53. Shields DC, Cheng ML, Flaherty AW, Gale JT, Eskandar EN. Microelectrode-guided deep brain stimulation for Tourette syndrome: within-subject comparison of different stimulation sites. Stereotact Funct Neurosurg. 2008; 86:87–91.

54. Ackermans L, Duits A, Temel Y, Winogrodzka A, Peeters F, Beuls EA, et al. Long-term outcome of thalamic deep brain stimulation in two patients with Tourette syndrome. J Neurol Neurosurg Psychiatry. 2010; 81:1068–1072.

55. Kaido T, Otsuki T, Kaneko Y, Takahashi A, Omori M, Okamoto T. Deep brain stimulation for Tourette syndrome: a prospective pilot study in Japan. Neuromodulation. 2011; 14:123–128. discussion 129.

56. Lee MW, Au-Yeung MM, Hung KN, Wong CK. Deep brain stimulation in a Chinese Tourette's syndrome patient. Hong Kong Med J. 2011; 17:147–150.
57. Savica R, Stead M, Mack KJ, Lee KH, Klassen BT. Deep brain stimulation in tourette syndrome: a description of 3 patients with excellent outcome. Mayo Clin Proc. 2012; 87:59–62.

58. Ackermans L, Temel Y, Cath D, van der, Bruggeman R, Kleijer M, et al. Deep brain stimulation in Tourette's syndrome: two targets? Mov Disord. 2006; 21:709–713.

59. Shahed J, Poysky J, Kenney C, Simpson R, Jankovic J. GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbidities. Neurology. 2007; 68:159–160.

60. Dehning S, Mehrkens JH, Müller N, Bötzel K. Therapy-refractory Tourette syndrome: beneficial outcome with globus pallidus internus deep brain stimulation. Mov Disord. 2008; 23:1300–1302.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download