Abstract
Background
Acquired neuromyotonia (NMT) forms part of the spectrum of acquired peripheral nerve hyperexcitability syndrome, and is thought to be caused by antibodies to voltage-gated potassium channels (VGKC). Exertional weakness is unusual unless autoimmune myasthenia gravis (MG) is superimposed.
Acquired neuromyotonia (NMT) is a rare condition of spontaneous and continuous muscle fiber activity that is thought to be caused by voltage-gated potassium channel (VGKC) antibodies that functionally block the neuronal potassium channels at the distal motor axons.1 Exertional weakness is unusual without coexistence of autoimmune myasthenia gravis (MG).1 A patient with NMT who developed exertional weakness without coexisting seropositive MG is reported herein.
A 19-year-old man presented with generalized muscle stiffness and cramps of 5 weeks duration. He also noticed moderate limb weakness, and complained of difficulty climbing stairs, elevating his arms, and handwriting. Repeated exertions relieved the stiffness, but made the weakness worse. He had paresthesia and hyperhidrosis in both hands and feet. Neurological examination revealed mild limb weakness with remarkable fatigability (Fig. 1A), diffuse fasciculation, and myokymia. Repetitive nerve stimulation (RNS) of the ulnar nerve at low frequencies revealed marked reductions in compound muscle action potential (CMAP) amplitudes (Fig. 1B), but there was neither postexercise facilitation nor abnormal increase at a stimulation frequency of 50 Hz. The findings of a conventional nerve conduction study were normal except for abnormal stimulation-induced afterdischarges (Fig. 1C and D). Needle electromyography revealed varying degrees of neuromyotonic (Fig. 1E) and myokymic discharges with normal motor unit potentials. Antibody tests against VGKC, acetylcholine (ACh) receptor, and muscle-specific kinase were all negative. Computed tomography of the chest revealed no evidence of thymus abnormalities. All other laboratory tests yielded no other unusual findings.
With a presumed diagnosis of seronegative generalized MG superimposed on NMT, this patient was treated with high-dose intravenous immunoglobulin (IVIg) along with oral carbamazepine and a low dose of pyridostigmine bromide (30 mg three times per day). Within 2 days, even before the IVIg treatment was completed, the patient had responded so dramatically that not only the muscle stiffness but also the weakness had improved to a near-premorbid state. However, a follow-up nerve conduction study at that time was not fully consistent with the clinical recovery, revealing the occurrence of repetitive CMAPs (repCMAPs) along with more prominent afterdischarges (Fig. 1F). The repCMAPs disappeared after discontinuation of pyridostigmine, but the abnormal afterdischarges persisted in the peroneal and tibial nerves. Abnormal CMAP decrements continued to be present in a follow-up RNS study, but to a lesser degree with larger CMAPs (Fig. 1G). The patient continued to receive carbamazepine treatment only, and his clinical improvement was maintained during the next 2 years of follow-up.
It was initially assumed that the patient had two separate conditions: NMT and MG. However, subsequent clinical and electrophysiological observations seemed to discount this, raising another possibility that the observed myasthenia was related to the pathophysiology of NMT. Indeed, it has been suggested that the constant and rapid motor unit firing in NMT reduces the amount of immediately releasable quanta in the nerve terminals, causing a decrease in the quantal content.1 The quantal response may also be impaired, since the prolonged and repeated exposure of ACh receptors to their agonist can result in prolongation of the endplate current, leading to receptor desensitization and depolarization block.2 The initially quite low CMAP amplitudes in the present patient may be explained by such defects in neuromuscular junction transmission in NMT. The rapid therapeutic response of the myasthenia in this patient, in terms of both clinical and electrophysiological aspects, could support this explanation.
The "neural" repCMAPs, also called M-wave afterdischarges, have been reported in two other cases with NMT.3 Unlike "synaptic" repCMAPs, "neural" repCMAPs are known to occur in the supernormal phase of repolarization, triggered at about 10 ms after the passage of an impulse.4,5 This is in contrast to synaptic repCMAPs, which begin 3.5-4.0 ms after the primary discharge.4 Interestingly, there were both "neural" and "synaptic" repCMAPs in our patient, with the latter observed after treatment with pyridostigmine at a very low dose. The occurrence of "synaptic" repCMAPs might be attributable to the pyridostigmine-induced additional excess of ACh in the synaptic space, which in our case was already fullycharged.
Previous findings are not consistent with the presence of neurophysiologically detectable neuromuscular transmission defects in patients with NMT.3,6,7 This discrepancy may be attributable to heterogeneity in the severity and extent of the pathological processes underlying NMT.1 Coincident autoimmune MG might have further complicated the issue of the origin and pathophysiology of exertional weakness in NMT. Although the mechanism involved remains to be elucidated, the findings of the present case report suggest that 1) exertional weakness can develop in NMT without coexisting autoimmune MG, and 2) the constantly rapidly firing motor units in NMT may compromise the safety factor at the neuromuscular junction sufficiently to cause a neuromuscular transmission defect.
Figures and Tables
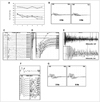 | Fig. 1Coexistence of myasthenia and neuromyotonia. A: Repeated measures of maximal handgrip strength demonstrating the presence of exertional muscle weakness. Strength was measured six times, with a 5-second rest between trials, revealing decreases of 30% and 55% on the right and left sides, respectively. The uppermost line was obtained from a healthy subject of the same sex and body weight. B: Low-frequency repetitive nerve stimulations of the ulnar nerve showing markedly abnormal reductions of compound muscle action potential (CMAP) amplitudes (28% and 43% at 2- and 3-Hz stimulation frequencies, respectively). C and D: Stimulation-induced M-wave and F/H-wave afterdischarges in the peroneal and tibial nerves. E: A 5-second continuous needle electromyogram recording from the biceps brachii muscle, showing high-frequency waning continuous motor unit discharges. F: Synaptic repetitive CMAP (arrow) along with prominent M-wave and F-wave afterdischarges at the abductor digiti minimi muscle after supramaximal stimulation of the ulnar nerve. G: Moderate reductions of CMAP amplitudes in response to lowfrequency repetitive nerve stimulation of the ulnar nerve at 1 week after treatment (19% and 15% at 2- and 5-Hz stimulation frequencies, respectively). Note the increased CMAPs and smaller decrements compared to those obtained before treatment (B). |
Acknowledgements
We are grateful to Dr. A. Vincent at the Weatherall Institute of Molecular Medicine in Oxford, UK, for her kind help in measuring the VGKC and muscle-specific kinase antibodies.
This study is supported by grant no.16-2013-116 from the SK Telecom Research Fund.
References
2. Auerbach A, Akk G. Desensitization of mouse nicotinic acetylcholine receptor channels. A two-gate mechanism. J Gen Physiol. 1998; 112:181–197.
3. Singh G, Avasthi G, Khurana SB. Continuous muscle fibre activity: description of neuroelectromyographic findings including single fibre EMG. Electromyogr Clin Neurophysiol. 1998; 38:121–127.
4. van Dijk JG, Lammers GJ, Wintzen AR, Molenaar PC. Repetitive CMAPs: mechanisms of neural and synaptic genesis. Muscle Nerve. 1996; 19:1127–1133.

5. Torbergsen T, Stålberg E, Brautaset NJ. Generator sites for spontaneous activity in neuromyotonia. An EMG study. Electroencephalogr Clin Neurophysiol. 1996; 101:69–78.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download