We describe herein the case of a 37-year-old woman who presented with slowly progressive lower-limb stiffness and gait ataxia. She complained of running difficulties since the age of 10 years, gait impairment since the age of 30 years, and urinary urgency during the past 2 years. Her family history was unremarkable. At the last examination she exhibited spastic gait, pyramidal signs, severe impairment of the vibration sense in the lower limbs, and the Romberg sign. She achieved a Spastic Paraplegia Rating Scale score of 9/52.
Blood tests were normal except for elevated serum levels of cholesterol (223 mg/dL, normal range 110-200 mg/dL) and thyroid-stimulating hormone (4.67 µUI/mL, normal range 0.45-3.50 µUI/mL). The patient was treated with levothyroxine for previous autoimmune thyroiditis. Infectious and autoimmune diseases, vitamin deficiencies, and genetic mutations associated with the most frequently occurring inherited ataxias and spastic paraplegias (SPGs) were excluded (i.e., Friedreich's ataxia, spinocerebellar ataxia types 1, 2, 3, and 6, and SPG4 and 7).
Brain MRI findings were normal, but spinal MRI revealed severe thinning of the dorsal medulla. Visual and brainstem auditory evoked potentials were normal, but motor evoked potentials exhibited prolonged central motor conduction times (CMCTs) from the abductor hallucis (26.5/26.3 ms for left/right) and normal CMCTs from the thenar eminence muscles. Somatosensory evoked potentials demonstrated elongated central conduction times on stimulation of the median and tibial nerves: N13-N20 interval, 15.5/14.6 ms for left/right; and N22-P37 interval, 25.8/24.6 ms for left/right. The patient's cortical response amplitudes were mildly reduced for stimulation at the upper limbs (1.4/1.0 µV for left/right), and severely reduced for stimulation at the lower limbs (0.9/0.5 µV for left/right). Her peripheral motor-sensory conduction times were preserved.
Magnetoencephalography (MEG) was conducted using a 306-channel helmet-shaped magnetoencephalograph (Neuromag Triux, Elekta Neuromag TRIUX, Elekta OY, Helsinki, Finland)1 with 1-Hz stimulation of the median, ulnar, and tibial nerves, to obtain somatosensory evoked fields (SEFs). For upper-limb stimulation, generators of the first cortical peak of the SEF (corresponding to N20) were identified and localized in the sensorimotor cortical areas, although the latency was increased (Fig. 1). No magnetic components were obtained for lower-limb stimulation.
The association between sensory ataxia and spastic paraplegia led us to suspect that this patient had SPG5: mutational screening for the gene encoding oxysterol-7-alpha hydroxylase (CYP7B1)2 was performed, and the serum level of 27-hydroxycholesterol was measured. SPG5 patients generally present with increased serum levels of 27-hydroxycholesterol due to a deficiency of the P450 family enzyme oxysterol-7-alpha-hydroxylase.3 Our patient carried two CYP7B1 missense mutations, both of which have been described previously: c.260G>T (p.G87V) and c.1250G>A (p.R417H).2,4 The unaffected father carried the c.260G>T mutation, and the mother carried the c.1250G>A mutation.
The diagnosis of SPG5 was confirmed by the finding of elevated serum 27-hydroxycholesterol (754.3 µg/L; assayed using isotope-dilution mass spectroscopy).3 The two parents of the patient had serum 27-hydroxycholesterol levels of 177.2 and 147.5 µg/L (range in 27 controls, 84.1-210.8 µg/L).
Spastic paraplegia type 5 has been clinically described with either a "pure" or "complex" phenotype. Mild sensory abnormalities have been described in pure hereditary SPGs, among which SPG4 is the most frequently occurring. Furthermore, 47-58% of pure hereditary SPG patients present with mild vibration-sense abnormalities without elongation of central somatosensory conduction times.5 Although several SPGs may present with vibration-sense abnormalities, this feature has only been consistently described in SPG5 patients (94-100%)2,4 in association with elongated somatosensory central conduction times.6
The data obtained in this study confirmed the presence of a severe clinical and neurophysiologic somatosensory impairment in SPG5 that is due to selective dorsal-column degeneration. Our MEG study revealed that the magnetic cortical representation is conserved for upper-limb stimulation and absent for lower-limb stimulation, in agreement with the occurrence of a "dying-back" dorsal-column degeneration.6 This finding should be confirmed in a larger cohort of SPG5 patients since the absence of evoked MEG signals has been observed even in normal healthy subjects. The neurophysiologic somatosensory abnormalities in our patient were more pronounced and diffuse than corticospinal abnormalities.
These results contribute to the definition of the SPG5 phenotype. Despite the rarity of SPG5, the refinement of clinical, biochemical, and neurophysiologic descriptions could improve early diagnoses of this condition. Moreover, serum levels of 27-hydroxycholesterol represent a possible biomarker for therapeutic trials.
Figures and Tables
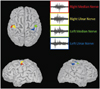 | Fig. 1Cortical representation of the first peak of the somatosensory evoked fields obtained by stimulation of the right median nerve (red), right ulnar nerve (yellow), left median nerve (green), and left ulnar nerve (blue). Source localization was performed using standardized weighted low-resolution electromagnetic tomography on individual MRI. |
References
1. Burgess RC, Funke ME, Bowyer SM, Lewine JD, Kirsch HE, Bagić AI, et al. American Clinical Magnetoencephalography Society Clinical Practice Guideline 2: presurgical functional brain mapping using magnetic evoked fields. J Clin Neurophysiol. 2011; 28:355–361.
2. Tsaousidou MK, Ouahchi K, Warner TT, Yang Y, Simpson MA, Laing NG, et al. Sequence alterations within CYP7B1 implicate defective cholesterol homeostasis in motor-neuron degeneration. Am J Hum Genet. 2008; 82:510–515.

3. Schüle R, Siddique T, Deng HX, Yang Y, Donkervoort S, Hansson M, et al. Marked accumulation of 27-hydroxycholesterol in SPG5 patients with hereditary spastic paresis. J Lipid Res. 2010; 51:819–823.

4. Arnoldi A, Crimella C, Tenderini E, Martinuzzi A, D'Angelo MG, Musumeci O, et al. Clinical phenotype variability in patients with hereditary spastic paraplegia type 5 associated with CYP7B1 mutations. Clin Genet. 2012; 81:150–157.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download