Abstract
Background and Purpose
Ocular manifestation is one of the frequent signs of an acute attack in multiple sclerosis (MS), although primary position upbeat nystagmus (PPUN) is rare. The purpose of this study is to determine the incidence of PPUN in MS and to determine the lesions that are responsible for this sign.
Methods
The medical records of 120 MS patients with acute brain lesions were reviewed over a consecutive period of 9 years; of these, 6 patients were found to have PPUN. Other ocular motor abnormalities were analyzed in combination with upbeat nystagmus, video-oculographic findings, and lesions detected on brain MRI.
Results
Lesions in the pontine tegmentum involving the medial longitudinal fasciculus (MLF) and ventral tegmental tract (VTT) were the most common, being observed in three of the six patients with PPUN. One patient exhibited caudal medullary lesions bilaterally affecting the paramedian portion of the posterior tegmentum, and two patients exhibited multiple lesions involving the pons with the cerebral peduncle or medulla. In five patients, other ocular motor dysfunctions, such as gaze-evoked nystagmus (n=3) and internuclear ophthalmoplegia (n=1), were found in combination with upbeat nystagmus.
Conclusions
PPUN is an infrequent, ocular manifestation noted during an acute attack of MS, and was observed in 5% of the present cases. Brainstem lesions in these cases primarily involved the pontine tegmentum and the caudal medulla. These findings support the theory that upbeat nystagmus is attributable to damage to the upward vestibulo-ocular reflex pathway related to the vestibular nucleus, VTT, and interconnecting pathways.
Ocular manifestation is one of the most frequent and important signs observed during an acute attack of multiple sclerosis (MS). Common abnormal eye movements include saccadic dysmetria, internuclear ophthalmoplegia (INO), impairment of the vestibulo-ocular reflex (VOR), and gaze-evoked nystagmus (GEN).1 However, primary position upbeat nystagmus (PPUN) is a rare manifestation in MS.
The amplitude of PPUN usually increases during upward gaze, with impaired vertical pursuit, but the amplitude decreases during downward gaze. It is neither enhanced during lateral gaze nor reduced by fixation, and should be distinguished from the more common GEN, which is observed only during upward gaze. PPUN can be caused by lesions involving the ventral tegmentum of the rostral medulla and the caudal pons, midbrain, brachium conjunctivum (BC), and cerebellum.2,3 In the lower medulla in particular, it is associated with lesions affecting the prepositus hypoglossi nucleus, vestibular nuclei, nucleus intercalatus, and flocculus. Upbeat nystagmus (UBN) is caused by damage to the interconnection between the structures controlling the vertical vestibulo-ocular pathways or vertical smooth pursuit,4 and it has been reported in patients with infarctions, demyelination,3 glioma,4 and Wernicke's encephalopathy.5
The aim of the present study was to determine the incidence of PPUN in acute attacks of MS and describe the anatomic correlation of UBN on brain MRI.
The medical records of patients who were admitted due to exacerbations of MS at Asan Medical Center between March 2000 and January 2009 were reviewed, and patients with acute MS lesions on brain MRI and who exhibited PPUN were enrolled. The diagnosis of MS was based on the 2010 McDonald criteria. PPUN was defined as UBN observed when resting and with stronger upbeat rather than horizontal or torsional components, in addition to increasing amplitude during upward gaze. Patients with vertical gaze-evoked UBN observed only during vertical gaze were excluded.
The following clinical details were obtained for each patient: sex, age, other ocular motor abnormalities, video-oculographic findings, and lesions seen on brain MRI. Brain MRI using a 1.5-tesla unit was performed in all patients within 1-28 days after the symptom onset. An axial T2-weighted scan [repetition time/echo time (TR/TE)=2,500/80 ms] was performed in the horizontal plane. T1-weighted (TR/TE=600/20 ms) axial and sagittal images were also obtained. The T2-weighted axial images were used for analysis. Eye movements were observed using video Frenzel goggles and were recorded using video-oculography (SLMED, Seoul, Korea).
Of the 156 consecutive patients with exacerbations of MS, 120 had acute lesions on brain MRI, of which 6 (5%) exhibited PPUN during straight-ahead gaze. All six were women, their mean age was 41 years (range, 20-57 years), and their mean disease duration was 3.5 years (range, 1-9 years). Table 1 summarizes the clinical and demographic data of these patients. Other oculomotor abnormalities were observed in 24 patients. The demographic and oculomotor findings of these patients are summarized in Table 2.
The most common lesions were pontine lesions involving the ventral tegmental tract (VTT) or medial longitudinal fasciculus (MLF); these were observed in three patients (patients 1-3). One patient (patient 6) had MS lesions in the dorsal part of the lower medulla that involved the nucleus intercalatus. Two patients had multiple brainstem lesions (i.e., pontine tegmentum with superior and middle cerebellar peduncle in patient 4, and diffuse lesions extending from the caudal pons to the dorsal rostral medulla in patient 5). In five patients (patients 1-5), UBN was accompanied by other ocular abnormalities such as horizontal GEN (n=3) and INO (n=2). Fig. 1. shows the brain MRI scans of the six MS patients with PPUN.
A 34-year-old woman presented with dizziness and dysphagia. Three years before the onset of her symptoms she had suffered from transverse myelitis at the T5-T9 level. A neuro-ophthalmological examination revealed PPUN but without weakness of the extraocular muscles. The amplitude and frequency of the UBN increased during upward gaze and decreased during downward gaze. Video-oculographic recording revealed that the nystagmus was upbeating in the primary position of gaze with a mean velocity of 7°/sec, and its slow phase was linear, with a constant velocity. The nystagmus changed to downbeating with a mean velocity of 26.6°/sec during rightward gaze and it disappeared during leftward gaze (Suppl. 1), and reversed direction to downbeat when lying down and during rightward and leftward head turning (Suppl. 2). The nystagmus was also reversed to downbeat during central head hanging, and right and left head hanging. The gag reflexes and the power of the tongue were also decreased. Motor and sensory changes were not reported in her extremities. Brain MRI revealed high T2 signal intensities in the dorsal paramedian area of the caudal medulla, and spinal MRI revealed no evidence of new lesions. The patient's symptoms gradually improved with steroid therapy. However, 20 days later she was seen again because of sudden-onset severe dizziness. Video-oculographic findings revealed downbeat nystagmus in the primary position, but without other alterations (Suppl. 3). The direction of the nystagmus did not change in any of the head positions. Follow-up brain MRI revealed no definite interval changes of the lower medullary lesion. The downbeat nystagmus persisted to the 1-month follow-up after discharge.
Ocular abnormalities can be initial manifestations of MS and may predict additional demyelinating events.6,7 Nevertheless, PPUN is an infrequent ocular manifestation during acute attacks of MS, as was observed in 5% of the present cases.
In the present study, the pons was the main anatomical location responsible for UBN (Fig. 1). In the ascending route of the upward VOR pathway, the signals from the anterior semicircular canals are sent to the superior vestibular nucleus (SVN) and then through the BC to the oculomotor nucleus in the midbrain via the VTT or MLF.8 In cases of UBN caused by a pontine lesion, the lesion is usually located in the posterior basis pontis or in the ventral tegmentum at the level of the rostral pons.2 In three of the patients in this study (patients 1-3), the demyelinating lesion was observed in the posterior part of the basis pontis at the level of the rostral pons. In one of these (patient 2), brain MRI revealed involvement of the VTT.
The VTT lies slightly ventral and lateral to the BC in the lower pons, arching medially and decussating above the level of the midpons, in the posterior part of the basis pontis.9 Therefore, UBN may arise from a small, unilateral lesion in the posterior basis pontis of the rostral pons. This patient also harbored midline MLF involvement. The MLF lesion may be another explanation for PPUN. Four patients (patients 1-3, and 5) possessed midline MLF lesions at the level of the rostral to caudal pons. PPUN as a result of MLF lesion is very rare; damage to the MLF mainly causes INO or skew deviation. However, a selective lesion in the upward pathway of the VOR with sparing of the downward pathway10 may result in UBN.
One case (patient 4) exhibited involvement of the BC on brain MRI. A few patients with UBN attributed to unilateral BC lesions have also been reported.11,12 The role of the BC could be considered since this structure is generally thought to transmit vertical slow eye movement signals to the oculomotor nucleus, so that theoretically it is possible for UBN to appear after BC damage.9
Brain MRI in patient 6 demonstrated dorsal caudal medullar lesions involving the nucleus intercalatus. The caudal medulla is linked to the visual pursuit system. The nucleus intercalatus, one of the components of the perihypoglossal nuclei, also receives excitatory signals from the SVN and has an interconnection with the prepositus hypoglossi nucleus.13 The prepositus hypoglossi nucleus receives afferent inhibitory signals from the cerebellar vermis and the flocculus, and then excites the oculomotor neurons.9 Therefore, UBN may result from lesions in these structures. Some authors have considered a lesion in the nucleus intercalatus of the medulla to be the most reasonable explanation for PPUN.14-16 In patient 6 in the present study, UBN changed to downbeat nystagmus in the primary position 20 days later, without definite changes in the caudal medullar lesions on brain MRI. There have only been a few case reports of UBN spontaneously changing to downbeat nystagmus in patients with Wernicke's encephalopathy5,17 and medullary hemorrhage.18 It has been reported that the caudal brainstem, and more specifically the midline cerebellum, and the nucleus prepositus hypoglossi control vertical smooth pursuit, and that vertical nystagmus can result from damage to these structures.4 Directional changes of vertical nystagmus in our patient are thought to be attributable to interruption of tonic balancing smooth pursuit by caudal medulla lesions.
As shown by the present illustrative case, UBN could be observed transiently, since the latency from the onset was also variable. One limitation of the present retrospective study was the evaluation of delicate and transient neuro-ophthalmologic findings, especially UBN.
In conclusion, the cases described herein support the theory that UBN is caused by damage to the ascending routes of the VOR pathway related to the VTT, SVN, and caudal medulla, and their interconnecting pathways, and are consistent with the results of previous studies. PPUN is not a common ocular manifestation in acute attacks of MS, but it can provide important clues regarding the location of brainstem lesions.
Figures and Tables
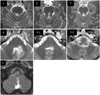 | Fig. 1Brain MRI scans of patients with upbeat nystagmus. Number on each figure represents patient's number. |
Table 1
Clinical profiles of the patients with primary position upbeat nystagmus (PPUN) in multiple sclerosis (MS)

Table 2
Clinical profiles of 24 patients with the other oculomotor abnormalities

B: bilateral, DN: downbeat nystagmus, GEN: gaze-evoked nystagmus, Hori: horizontal, ICP: inferior cerebellar peduncle, INO: internuclear ophthalmoplegia, L: left, MCP: middle cerebellar peduncle, med.: medulla, MLF: longitudinal fasciculus, PAG: periaqueductal gray matter, pont.: pontine, R: right, ro: rostal, SCP: superior cerbellar peduncle, teg.: tegmentum, Ver.: vertical.
References
1. Derwenskus J, Rucker JC, Serra A, Stahl JS, Downey DL, Adams NL, et al. Abnormal eye movements predict disability in MS: two-year follow-up. Ann N Y Acad Sci. 2005; 1039:521–523.

2. Fisher A, Gresty M, Chambers B, Rudge P. Primary position upbeating nystagmus. A variety of central positional nystagmus. Brain. 1983; 106:949–964.
3. Hirose G, Kawada J, Tsukada K, Yoshioka A, Sharpe JA. Upbeat nystagmus: clinicopathological and pathophysiological considerations. J Neurol Sci. 1991; 105:159–167.

4. Gilman N, Baloh RW, Tomiyasu U. Primary position upbeat nystagmus. A clinicopathologic study. Neurology. 1977; 27:294–298.

5. Cox TA, Corbett JJ, Thompson HS, Lennarson L. Upbeat nystagmus changing to downbeat nystagmus with convergence. Neurology. 1981; 31:891–892.

6. Chen L, Gordon LK. Ocular manifestations of multiple sclerosis. Curr Opin Ophthalmol. 2005; 16:315–320.

7. Solingen LD, Baloh RW, Myers L, Ellison G. Subclinical eye movement disorders in patients with multiple sclerosis. Neurology. 1977; 27:614–619.

8. Ranalli PJ, Sharpe JA. Upbeat nystagmus and the ventral tegmental pathway of the upward vestibulo-ocular reflex. Neurology. 1988; 38:1329–1330.

9. Pierrot-Deseilligny C, Milea D. Vertical nystagmus: clinical facts and hypotheses. Brain. 2005; 128:1237–1246.

10. Kim JS, Yoon B, Choi KD, Oh SY, Park SH, Kim BK. Upbeat nystagmus: clinicoanatomical correlations in 15 patients. J Clin Neurol. 2006; 2:58–65.

11. Benjamin EE, Zimmerman CF, Troost BT. Lateropulsion and upbeat nystagmus are manifestations of central vestibular dysfunction. Arch Neurol. 1986; 43:962–964.

12. Kattah JC, Dagi TF. Compensatory head tilt in upbeating nystagmus. J Clin Neuroophthalmol. 1990; 10:27–31.
13. Ohkoshi N, Komatsu Y, Mizusawa H, Kanazawa I. Primary position upbeat nystagmus increased on downward gaze: clinicopathologic study of a patient with multiple sclerosis. Neurology. 1998; 50:551–553.

14. Saito T, Aizawa H, Sawada J, Katayama T, Hasebe N. Lesion of the nucleus intercalatus in primary position upbeat nystagmus. Arch Neurol. 2010; 67:1403–1404.

15. Munro NA, Gaymard B, Rivaud S, Majdalani A, Pierrot-Deseilligny C. Upbeat nystagmus in a patient with a small medullary infarct. J Neurol Neurosurg Psychiatry. 1993; 56:1126–1128.

16. Adamec I, Gabelić T, Krbot M, Ozretić D, Milivojević I, Habek M. Primary position upbeat nystagmus. J Clin Neurosci. 2012; 19:161–162.

SUPPLEMENTARY MATERIALS
Supplemental Video Clip. 1
Video-oculography of patient 6. (a) Upbeating nystagmus in the primary position. (b) During rightward gaze the nystagmus was changed to downbeating with a mean velocity of 26.6°/sec. (c) During leftward gaze the upbeat nystagmus disappeared.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download