Abstract
Background
Patients who develop horizontal and vertical saccadic palsy after cardiac surgery have rarely been described. Although most such patients exhibit distinct neurological deficits, their brain MRI findings are almost normal. In addition, functional neuroimaging of such patients has never been reported.
Case Report
A 43-year-old woman with dysarthria, dysphagia, and horizontal and vertical saccadic palsy after cardiac surgery was followed up for about 6 years; serial brain MRIs has been performed during this period, including susceptibility-weighted imaging (SWI) and [18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET). Multiple microbleeds in the cerebral cortex, cerebellum, and brainstem, and glucose hypometabolism in the brainstem, cerebellum, and multiple cortical areas.
There are few reports of patients who develop neurological deficits after cardiac surgery in the literature.1,2,3,4,5 Although there are differences in the clinical manifestations of each case, most patients exhibit both horizontal and vertical saccadic palsy.
Based on accompanying symptoms such as dysarthria, gait disturbance, and dysphagia, it has been speculated that the main neural correlate producing these unique phenomena is the brainstem, including the paramedian pons and the midbrain. However, few definite abnormalities on neuroimaging have been described.1 We report herein a patient who presented with dysarthria, dysphagia, and horizontal and vertical saccadic palsy after cardiac surgery, with serial brain MRI and [18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) and the clinical course over a 6-year period.
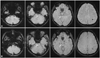
A 43-year-old woman with multiple traumatic injuries including traumatic aortic dissection due to a traffic accident was admitted to hospital. She underwent uncomplicated thoracic aorta replacement under deep hypothermia with circulatory arrest 18 days after the accident. Two days after the operation she complained of difficulty in swallowing and articulation, and tunnel vision. A neurological examination revealed both horizontal and vertical slow saccades with preserved smooth pursuit and vestibulo-ocular reflex (Supplementary Video 1), dysarthria, dysphagia, impaired tongue protrusion, and bilateral tilting on tandem gait. Horizontal and vertical quick phases were not elicited when using an optokinetic drum. The initial brain MRI findings were normal; however, [18F]-FDG-PET performed 1 year postonset demonstrated severe glucose hypometabolism in the brainstem, cerebellum, and bilateral medial temporal and parietal areas (Fig. 1A). Her symptoms persisted without improvement for the following 4 years. Visuospatial dysfunction and impaired verbal memory were detected by neuropsychological tests performed at 4 years postoperatively. Repeated MRI disclosed multiple microbleeds in the midbrain, pons, left cerebellum, and left parietal lobe on susceptibility-weighted imaging (SWI) (Fig. 2A). Follow-up [18F]-FDG-PET demonstrated severe glucose hypometabolism in the initially affected regions (Fig. 1B). One year later, only her swallowing difficulty and cognitive dysfunction had slightly improved. The third series of MRI and [18F]-FDG-PET performed approximately 6 years after the surgery continued to show multiple microbleeds and severe glucose hypometabolism in the previously affected regions (Figs. 1C and 2B).
The most common finding in patients with neurological deficits after cardiac surgery is selective horizontal and vertical saccadic palsy.1,2,3,4,5 Two types of saccadic generator have been reported. First, premotor excitatory burst neurons (PBNs) in the paramedian pontine reticular formation at the level of the abducens nuclei generate horizontal saccades, while PBNs in the rostral interstitial nuclei of the medial longitudinal fasciculus of the midbrain produce vertical saccades.1,4 Second, omnipause neurons (OPNs) in the midline of the pontine raphe interpositus nucleus, which normally inhibit both types of PBN, enhance saccadic generation through a temporary pause when a saccade is initiated.1,4 Experimentally induced lesions of OPNs induce slow horizontal and vertical saccades.4 Our patient developed both horizontal and vertical saccadic palsy, similar to those reported in other patients, and so we hypothesized that the OPNs were damaged, possibly by ischemic injury due to hypotension or microembolism during the operation. One autopsy-confirmed case exhibited focal neuronal loss and gliosis confined to the median and paramedian pons where OPNs reside.6 However, most published cases did not exhibit brainstem abnormalities on brain MRI or computed tomography, and only one patient who developed saccadic palsy after aortic valve replacement demonstrated a focal small lesion in the dorsomedial pons on brain MRI.1
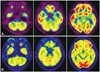
There are no previous reports of functional imaging findings. Although we did not compare our patient to age-matched healthy controls, glucose hypometabolism in the brainstem was clearly identifiable by visual inspection when compared to an age-matched female patient with Alzheimer's disease (Fig. 3). The patient's first MRI findings (not including SWI) were normal, but SWI performed at 4 and 6 years after onset demonstrated multiple microbleeds in the brainstem, cerebellum, and cerebral hemisphere. A recent T2*-weighted gradient-echo imaging study carried out in patients who underwent cardiac valve surgery showed that 12 out of 26 patients developed new lesions with cerebral microbleeds, and 2 of these exhibited transient neurological deficits.7
It is unclear precisely how the cerebral microbleeds on SWI were associated with the clinical symptoms of our patient. However, glucose hypometabolism in the brainstem, cerebellum, and multiple cortical areas detected by [18F]-FDG-PET in our patient was at least partially compatible with microbleed-induced lesions and the clinical symptoms of our patient, including saccadic palsy, dysphagia, dysarthria, gait ataxia, and cognitive deficits.
Collectively, the findings of this case underscore the need for further studies to examine the clinical implications of microbleeds on SWI and glucose hypometabolism on [18F]-FDG-PET after cardiac surgery. In addition, voxel-based analysis using statistical parametric mapping of functional imaging should be performed to confirm the presence of decreased brainstem glucose metabolism in patients with selective saccadic palsy after cardiac surgery.
Figures and Tables
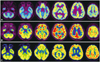 | Fig. 1Decreased glucose metabolism throughout the brainstem, cerebellum, and bilateral medial temporal and left parietal areas was detected by axial [18F]-fluoro-2-deoxy-D-glucose positron-emission tomography performed 1 year (A), 4 years (B), and approximately 6 years (C) after cardiac surgery. |
Acknowledgements
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C 2020), and a clinical research grant from Pusan National University Hospital 2013.
References
1. Eggers SD, Moster ML, Cranmer K. Selective saccadic palsy after cardiac surgery. Neurology. 2008; 70:318–320.

2. Kim EJ, Oh SY, Choi HC, Shin BS, Seo MW, Choi JB. Selective saccadic palsy after cardiac surgery. J Neuroophthalmol. 2010; 30:268–271.

3. Mokri B, Ahlskog JE, Fulgham JR, Matsumoto JY. Syndrome resembling PSP after surgical repair of ascending aorta dissection or aneurysm. Neurology. 2004; 62:971–973.

4. Solomon D, Ramat S, Tomsak RL, Reich SG, Shin RK, Zee DS, et al. Saccadic palsy after cardiac surgery: characteristics and pathogenesis. Ann Neurol. 2008; 63:355–365.

5. Tomsak RL, Volpe BT, Stahl JS, Leigh RJ. Saccadic palsy after cardiac surgery: visual disability and rehabilitation. Ann N Y Acad Sci. 2002; 956:430–433.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download