Abstract
Background and Purpose
To determine the correlation between the cross-sectional area (CSA) of the median nerve measured at the wrist using three-dimensional (3D) ultrasonography (US) and the electrophysiological severity of carpal tunnel syndrome (CTS).
Methods
We prospectively examined 102 wrists of 51 patients with clinical CTS, which were classified into 3 groups according to the electrodiagnostic (EDX) findings. Median nerve CSAs were measured using 3D US at the carpal tunnel inlet and at the level of maximal swelling.
Results
Ten wrists were negative for CTS. Of the 92 CTS-positive wrists, 23, 30, and 39 were classified as having mild, moderate, and severe CTS, respectively. The median nerve CSA differed significantly between the severe- and moderate-CTS groups (p=0.0007 at the carpal tunnel inlet and p<0.0001 at the maximal swelling site). There was a correlation between median nerve CSA and EDX parameters among those wrists with severe and mild CTS (p<0.0001 at both sites).
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy of the peripheral nerves,1 and is routinely diagnosed based on the clinical history, a physical examination, and electromyography.2,3 Ultrasonography (US) is currently emerging as a tool for diagnosing entrapment neuropathy of the upper extremities4,5 it is noninvasive, painless, readily available, and poses no radiation hazard. Several studies have investigated the usefulness of US in the diagnosis of CTS,3,6,7,8,9,10,11 most of which have produced a key finding of focal swelling of the median nerve at the carpal tunnel.10 Given the recent advances in the field of US, this tool has the potential to provide useful information regarding the detection and grading of CTS.
Advances in US imaging have resulted in the development of three-dimensional (3D) US, which has been particularly useful in the field of obstetrics.12 A probe has been developed for use with 3D US for the evaluation of peripheral nerves.13 One study that explored the assessment of CTS using 3D US and compared the cross-sectional area (CSA) of the median nerve between controls and patients with CTS13 found the mean median nerve CSA was significantly greater in the patient group than in the control group. However, few studies have evaluated whether the median nerve CSA measured using 3D US is significantly correlated with the severity of CTS. If nerve swelling measured by US can be correlated with the electrophysiological severity of this disorder, this could be used in the development of a useful tool for screening CTS and for assessing the prognosis.
The aim of this study was to determine whether there is a correlation between the maximal CSA of the median nerve at the wrist measured using 3D US and the electrophysiological severity of CTS.
Fifty-one consecutive patients (102 wrists) who were referred to our department from orthopedic and plastic surgery clinics between May 2008 and February 2009 with a clinical diagnosis of bilateral CTS were enrolled as subjects in this study. The clinical inclusion criteria were presence of 1) paresthesia or numbness in the area innervated by the median nerve, and 2) a sensory deficit in the same area, or 3) muscle weakness or atrophy of the abductor pollicis brevis (Quality Standards Subcommittee of the American Academy of Neurology, 1993). The exclusion criteria were 1) a history of underlying diseases such as generalized inflammatory polyneuropathy, gout, rheumatoid arthritis, and metabolic disorders, 2) pregnancy, 3) previous surgery for a fracture or laceration at the wrist, or 4) other lesions within the carpal tunnel. Review board approval was obtained for this study, and informed consent to participate was provided by all of the subjects.
A nerve conduction study (NCS) was conducted using a Dantec Counterpoint MK2 device (Medtronic, Minneapolis, MN, USA). All studies were conducted at standard room temperature (25℃). The skin temperature of the hand was maintained at 32℃ or above. The median compound muscle action potentials (CMAPs) were recorded over the abductor pollicis brevis muscle via median nerve stimulation applied 8 cm proximal to the active recording electrode. The onset latency and baseline-to-peak amplitude of the CMAPs were measured. The median sensory nerve action potentials (SNAPs) were recorded antidromically with a bar electrode over the third digit, and stimulated at two points located 7 and 14 cm proximal to the active recording electrode. To test the transcarpal segment, the nerve was also stimulated at two points: at the distal wrist crease (DWC) and 5 cm distal to the DWC at the palm. The latencies and baseline-to-peak amplitudes of the median SNAPs were measured and the onset latency difference between the two points was calculated. Needle electromyography was performed to exclude cervical radiculopathy.
Carpal tunnel syndrome was diagnosed based on the following criteria: 1) median SNAP peak latency of >3.7 ms, 2) SNAP peak latency longer in the proximal 7-cm segment than in the distal 7-cm segment, 3) SNAP amplitude <20 µV and conduction block with a SNAP amplitude decrease of >50% with wrist stimulation compared to palm stimulation, 4) 5-cm transcarpal conduction time of >1.3 ms, 5) median CMAP distal latency of >4.2 ms, and 6) CMAP amplitude of <4.5 mV. CTS in those wrists that tested positive for the disorder (n=92) was graded as mild, moderate, or severe based on a modification of the criteria given by Stevens (1997) as follows:
1) Mild CTS-prolonged (relative or absolute) sensory or mixed nerve action potential (NAP) distal latency (orthodromic, antidromic, or palmar) with or without SNAP amplitude below the lower normal limit.
2) Moderate CTS-abnormal median sensory latencies as above, and (relative or absolute) prolongation of the median motor CMAP distal latency.
3) Severe CTS-prolonged median CMAP and SNAP distal latencies, with either an absent SNAP or mixed NAP, or a low-amplitude or absent thenar CMAP. Needle examination often reveals fibrillations, reduced recruitment, and changes in the motor unit potential.
Examinations were conducted using 3D US with a dedicated 8- to 15-MHz mechanical 3D-volume transducer (Logiq 9, GE Medical Systems, Milwaukee, WI, USA). US examinations were conducted on the same day as the electromyography. All patients were evaluated in a supine position with the palm up and the fingers semiextended. A 3D volume transducer was placed at the DWC, which enabled sufficient demonstration of long-axis images of the median nerve in the proximal carpal tunnel (Fig. 1).
A musculoskeletal radiologist (C.H.K.) with 10 years of US experience on a 3D workstation (Centricity Radiology RA 600 version 7.0, GE Medical Systems, Milwaukee, WI, USA) and who was blinded to the NCS findings reviewed the 3D scans using the same analysis methods as employed in our previous study.13 The morphology of the median nerve from the distal radius to the proximal carpal tunnel was assessed in three orthogonal planes that could be displayed along with graphic lines and dots to clearly demonstrate how each section projection cuts the volume (Fig. 2). The changes in the shape of the median nerve were evaluated qualitatively, and whether or not the points of maximum nerve swelling could be visualized using this process was established. The median nerve CSA was then measured, and morphological evaluation was performed using the 3D volume data. Using an indirect technique, the median nerve CSA was measured at the carpal tunnel inlet (scaphoid-pisiform level) and at the point of maximum swelling in cases for which it could be demonstrated (Fig. 2). The formula of an ellipsoid area (D1×D2×3.14/4) was used in this measurement. The measurements were repeated three times, and the average was used for statistical analysis.
The maximal CSA of the median nerve around the wrist was selected, and the Pearson coefficient for the correlation between the maximal median nerve CSA and the electrophysiological severity of the CTS was measured. All continuous variables were normally distributed, as established by the Kolmogorov-Smirnov test, and were adjusted for age and sex. They were also adjusted for using both hands of one patient in testing. Statistical analysis was conducted using repetitive ANOVA to test the differences between the means of the groups. The cutoff for statistical significance was set at p<0.05, and the rho score was also recorded. Statistical analyses were conducted using SPSS (version 15.0, SPSS Inc., Chicago, IL, USA).
In total, 102 wrists of 51 patients were assessed, of which 92 wrists were confirmed electrophysiologically as being affected by CTS; the remaining 10 wrists were negative for CTS. Both wrists of each patients were examined. Ultimately, 92 affected wrists in 50 patients (5 males and 45 females) were included in the study. The age of the patients was 55.6±8.1 years (mean±SD; range, 31-73 years). Bilateral CTS was present in 42 of the patients (Table 1).
Of the 92 affected wrists, 23, 30, and 39 were classified electrophysiologically with mild, moderate, and severe CTS. The overall median nerve CSA at the tunnel inlet was 13.2±6.2 mm2 in the 92 wrists: the values were 10.5±4.6, 11.6±2.6, and 17.1±7.5 mm2 in mild, moderate, and severe CTS, respectively; these values differed significantly between severe and moderate CTS (p=0.0007) and between severe and mild CTS (p<0.0001) (Fig. 3). The overall median nerve CSA at the level of maximal swelling was 14.9±6.6 mm2 in all 92 wrists; the values were 11.7±4.7, 13.0±3.4, and 19.7±7.2 mm2 in mild, moderate, and severe CTS, respectively; these values also differed significantly between severe and moderate CTS (p<0.0001) and between severe and mild CTS (p<0.0001) (Fig. 3).
The following variables were correlated with the median nerve CSA at the carpal tunnel inlet: distal CMAP latency (r=0.411, p<0.01), CMAP amplitude (r=-0.323, p<0.01), distal SNAP latency (r=0.343, p<0.01), and SNAP amplitude (r=-0.335, p<0.01). The following variables were correlated with the median nerve CSA at the level of maximal swelling: distal CMAP latency (r=0.469, p<0.01), CMAP amplitude (r=-0.359, p<0.01), distal SNAP latency (r=0.272, p<0.05), and SNAP amplitude (r=-0.265, p<0.05) (Table 2).
In our previous study we applied the 3D US approach to median nerve CSA measurements and demonstrated a significant difference between controls and patients with CTS.13 In the present study we compared the median nerve CSA as measured by 3D US in patients who had been classified as having mild, moderate, or severe CTS based on electrophysiological measurements. A significant difference in the CSA was found at both measured sites of the median nerve between the severe-CTS group and other two groups, but not between the mild- and moderate-CTS groups (Fig. 3).
Several previous studies used two-dimensional (2D) US to examine the relationship between the median nerve CSA and CTS severity. Bayrak et al.14 showed that the median nerve CSA at the wrist increased as the number of motor units of the abductor pollicis brevis muscles decreased. Padua et al.10 and Karadağ et al.15 also found that in CTS patients, median nerve CSA was positively correlated with the electrophysiological severity of their condition.
Recent technical developments have resulted in 3D US emerging as a new imaging tool. Conventional 2D US requires the operator to reconstruct a series of axial images, which is time-consuming; in contrast, since 3D US requires the acquisition of only a single volume, the examination is both less operator-dependent and less time-consuming (potentially less than 1 min).13 Given these advantages, the use of 3D US has become more widespread in the fields of obstetrics, cardiology, and vascular medicine. It provides a highly detailed and repeatable method for evaluating anatomical structures and disease entities.
Recently there has been a rapid increase in interest in the use of 3D US to examine nerves. With 2D US, only longitudinal and axial views can be obtained by rotating the transducer through 90°. In contrast, 3D US makes it possible to also acquire a coronal view.16 Choquet and Capdevila17 reported a case in which the catheter tip used for perineural block was incorrectly placed in the extraneural space when using 2D US; after withdrawing the catheter tip and confirming the target position using 3D US, it was successfully placed in the perineural space. It is thus clear that 3D US can provide improved imaging of structures and their surrounding tissues.
Furthermore, Girtler et al.18 showed that 3D US of the upper-extremity nerves was feasible for both qualitative evaluations (in terms of image quality, atypical nerve location, and findings potentially associated with compression neuropathy) and quantitative (CSA measurements) evaluations of the median, ulnar, and radial nerves in healthy volunteers. There were no differences in the median nerve CSA measurements made by two different operators, and there was a difference of only 2 mm2 in those made at each level along the nerve.18 Karmakar et al.16 also reported that the sciatic nerve could be imaged using 3D US. These results suggest that 3D US provides more detailed anatomical information about the surrounding structures than 2D US. In addition, the perineural space of the sciatic nerve can be identified distinctly, which may be helpful for targeting nerve blocks.16 There have been additional reports describing the use of 3D US for imaging the upper-extremity nerves. Clendenen et al.19 reported that 3D US could be used to image the infraclavicular brachial plexus, thus enabling targeting of the placement of an infraclavicular blockade. However, despite these reports of the use of 3D US to image nerves, this approach has not been widely adopted for assessing the median nerve in CTS. Given that CTS is the most common compressive neuropathy of the upper limb, 3D US is a potentially valuable approach.
Our findings using 3D US regarding the relationship between median nerve CSA and the neurophysiological severity of CTS were highly consistent with the previously reported findings obtained using 2D US. There was a gradual increase in CSA with increasing CTS severity on NCSs (Fig. 3). In general, CTS is correlated with axonal loss and increased water content following nerve swelling. Therefore, there may be a positive correlation between median nerve CSA and electrophysiological severity.10 Table 2 lists the correlations between electrodiagnostic (EDX) parameters and the median nerve CSAs at the inlet of the carpal tunnel and at the level of maximal swelling. Moran et al.20 found significant correlations between the median nerve CSA and the proportion of nonresponses in the NCS (r=0.61). Moreover, Lee et al.21 showed that US findings regarding the median nerve CSA at the carpal tunnel inlet were strongly correlated with the NCS findings in various forms of CTS. Specifically, they showed that proximal swelling of the median nerve was primarily correlated with the NCS data, positively correlated with sensory latency (r=0.47), and negatively correlated with SNAP amplitude (r=-0.50), CMAP amplitude (r=-0.46), motor nerve conduction velocity (r=-0.44), and sensory nerve conduction velocity (r=-0.60). Similar statistically significant correlations were observed in the present 3D US study (Table 2).
The present findings also show that 3D US could be used to accurately differentiate between the median nerve CSAs of patients with severe CTS and those with mild or moderate CTS, but not between the mild- and moderate-CTS groups (Fig. 3). Mondelli et al.22 demonstrated that there were no anomalies in 23.5% of mild cases using US. Furthermore, Moghtaderi et al.23 suggested that 2D US can provide good sensitivity and specificity in patients with at least a moderate degree of CTS, and Ooi et al.24 also reported that 2D US may have diagnostic potential for CTS cases with a median nerve CSA of >12.8 mm2, corresponding to at least a moderate degree of CTS. These previous reports could explain the present finding of a significant difference only between the severe-CTS group and the other two groups.
Certain limitations should be considered when interpreting the results of this study. First, a control group was not included; however, in a previous study we confirmed that there was a significant difference between control and CTS groups. In the present study we focused on the reliability of using 3D US to differentiate between CTS of various severities based on EDX. A second limitation is that there was no comparison of the diagnostic accuracy between 2D US and 3D US examinations. This issue should be addressed in future studies.
In conclusion, 3D US exhibited significant reliability for detecting CTS severity and could provide additional diagnostic value for this common peripheral nerve entrapment neuropathy.
Figures and Tables
Fig. 2
Multiplanar display of a 3D ultrasonography volume showing the median nerve (dots) in three orthogonal orientations. The planes are interactive and permit a complete tracing of the median nerve from the distal radius to the proximal carpal tunnel.
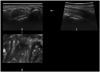
Fig. 3
Box plot showing the median nerve CSAs measured using 3D ultrasonography at the carpal tunnel inlet (left) and the maximal swelling point (right) in patients with CTS who were stratified according to the severity of their condition based on EDX (NCS). A gradual increase in CSA was observed with worsening CTS severity, with significant differences being apparent at both nerve sites between the severe- and mild-CTS groups (*p<0.0001 at the carpal tunnel inlet; †p<0.0001 at the maximum swelling point) and between the severe- and moderate-CTS groups (‡p=0.0007 at the carpal tunnel inlet; §p<0.0001 at the maximum swelling point). Bold black lines indicate medians and whiskers indicate error bars; ○, outliers; ■, mean values (10.5±4.6, 11.6±2.6, and 17.1±7.5 mm2 in the mild-, moderate-, and severe-CTS groups, respectively, at the carpal tunnel inlet; and 11.7±4.7, 13.0±3.4, and 19.7±7.2 mm2, respectively, at the maximum swelling point). CSAs: cross-sectional areas, CTS: carpal tunnel syndrome, EDX: electrodiagnostic, NCS: nerve conduction study.

References
1. Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999; 282:153–158.

2. Phalen GS. The carpal-tunnel syndrome. Clinical evaluation of 598 hands. Clin Orthop Relat Res. 1972; 83:29–40.
3. Lee D, van Holsbeeck MT, Janevski PK, Ganos DL, Ditmars DM, Darian VB. Diagnosis of carpal tunnel syndrome. Ultrasound versus electromyography. Radiol Clin North Am. 1999; 37:859–872. x
4. Buchberger W, Schön G, Strasser K, Jungwirth W. High-resolution ultrasonography of the carpal tunnel. J Ultrasound Med. 1991; 10:531–537.

5. Wiesler ER, Chloros GD, Cartwright MS, Smith BP, Rushing J, Walker FO. The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg Am. 2006; 31:726–732.

6. Duncan I, Sullivan P, Lomas F. Sonography in the diagnosis of carpal tunnel syndrome. AJR Am J Roentgenol. 1999; 173:681–684.

7. Wong SM, Griffith JF, Hui AC, Tang A, Wong KS. Discriminatory sonographic criteria for the diagnosis of carpal tunnel syndrome. Arthritis Rheum. 2002; 46:1914–1921.

8. Koyuncuoglu HR, Kutluhan S, Yesildag A, Oyar O, Guler K, Ozden A. The value of ultrasonographic measurement in carpal tunnel syndrome in patients with negative electrodiagnostic tests. Eur J Radiol. 2005; 56:365–369.

9. Ziswiler HR, Reichenbach S, Vögelin E, Bachmann LM, Villiger PM, Jüni P. Diagnostic value of sonography in patients with suspected carpal tunnel syndrome: a prospective study. Arthritis Rheum. 2005; 52:304–311.

10. Padua L, Pazzaglia C, Caliandro P, Granata G, Foschini M, Briani C, et al. Carpal tunnel syndrome: ultrasound, neurophysiology, clinical and patient-oriented assessment. Clin Neurophysiol. 2008; 119:2064–2069.

11. Klauser AS, Halpern EJ, De Zordo T, Feuchtner GM, Arora R, Gruber J, et al. Carpal tunnel syndrome assessment with US: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology. 2009; 250:171–177.

12. Dückelmann AM, Kalache KD. Three-dimensional ultrasound in evaluating the fetus. Prenat Diagn. 2010; 30:631–638.

13. Pyun SB, Kang CH, Yoon JS, Kwon HK, Kim JH, Chung KB, et al. Application of 3-dimensional ultrasonography in assessing carpal tunnel syndrome. J Ultrasound Med. 2011; 30:3–10.

14. Bayrak IK, Bayrak AO, Tilki HE, Nural MS, Sunter T. Ultrasonography in carpal tunnel syndrome: comparison with electrophysiological stage and motor unit number estimate. Muscle Nerve. 2007; 35:344–348.

15. Karadağ YS, Karadağ O, Ciçekli E, Oztürk S, Kiraz S, Ozbakir S, et al. Severity of Carpal tunnel syndrome assessed with high frequency ultrasonography. Rheumatol Int. 2010; 30:761–765.

16. Karmakar M, Li X, Li J, Sala-Blanch X, Hadzic A, Gin T. Three-dimensional/four-dimensional volumetric ultrasound imaging of the sciatic nerve. Reg Anesth Pain Med. 2012; 37:60–66.

17. Choquet O, Capdevila X. Case report: Three-dimensional high-resolution ultrasound-guided nerve blocks: a new panoramic vision of local anesthetic spread and perineural catheter tip location. Anesth Analg. 2013; 116:1176–1181.
18. Girtler MT, Krasinski A, Dejaco C, Kitzler HH, Cui LG, Sherebrin S, et al. Feasibility of 3D ultrasound to evaluate upper extremity nerves. Ultraschall Med. 2013; 34:382–387.

19. Clendenen SR, Robards CB, Clendenen NJ, Freidenstein JE, Greengrass RA. Real-time 3-dimensional ultrasound-assisted infraclavicular brachial plexus catheter placement: implications of a new technology. Anesthesiol Res Pract. 2010; 2010:pii:208025.

20. Moran L, Perez M, Esteban A, Bellon J, Arranz B, del Cerro M. Sonographic measurement of cross-sectional area of the median nerve in the diagnosis of carpal tunnel syndrome: correlation with nerve conduction studies. J Clin Ultrasound. 2009; 37:125–131.

21. Lee CH, Kim TK, Yoon ES, Dhong ES. Correlation of high-resolution ultrasonographic findings with the clinical symptoms and electrodiagnostic data in carpal tunnel syndrome. Ann Plast Surg. 2005; 54:20–23.

22. Mondelli M, Filippou G, Gallo A, Frediani B. Diagnostic utility of ultrasonography versus nerve conduction studies in mild carpal tunnel syndrome. Arthritis Rheum. 2008; 59:357–366.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download