Abstract
Background and Purpose
Whether or not migraine can cause cumulative brain alterations due to frequent migraine-related nociceptive input in patients is largely unclear. The aim of this study was to characterize longitudinal changes in brain activity between repeated observations within a short time interval in a group of female migraine patients, using resting-state functional magnetic resonance imaging.
Methods
Nineteen patients and 20 healthy controls (HC) participated in the study. Regional homogeneity (ReHo) and functional interregional connectivity were assessed to determine the focal and global features of brain dysfunction in migraine. The relationship between changes in headache parameters and longitudinal brain alterations were also investigated.
Results
All patients reported that their headache activity increased over time. Abnormal ReHo changes in the patient group relative to the HC were found in the putamen, orbitofrontal cortex, secondary somatosensory cortex, brainstem, and thalamus. Moreover, these brain regions exhibited longitudinal ReHo changes at the 6-week follow-up examination. These headache activity changes were accompanied by disproportionately dysfunctional connectivity in the putamen in the migraine patients, as revealed by functional connectivity analysis, suggesting that the putamen plays an important role in integrating diverse information among other migraine-related brain regions.
Migraine is an idiopathic headache disorder defined as an episodic disturbance primarily manifesting as head pain and touch sensitivity, accompanied by nausea and light sensitivity.1 Neuroimaging studies of migraineurs have described functional abnormalities in brain regions associated with pain processing as a result of repeated headache attacks, including enhanced cortical excitability2 and altered pain modulatory systems.3,4,5 However, the exact process underlying these brain abnormalities has not been fully elucidated, and whether or not migraine causes cumulative dysfunctional activities when the headache activity increases is still largely unknown.
Due to frequent migraine-related nociceptive input, several brain regions in migraine patients (PM) have been reported to exhibit abnormal functional activity at rest,6 leading to irregular brain circuits associated with pain-related information processing. Moreover, some studies patients have classified into two subgroups according to their migraine attack frequency and attack history.3,7 Comparisons between the milder and more severe patient groups were aimed at exploring the brain functional changes underlying the pathophysiology of migraine progression. While these studies have to some degree quantitatively identified the predilection site of possible brain functional changes in migraine, longitudinal studies are still needed. Accordingly, the aim of the present study was to characterize the longitudinal brain functional abnormalities occurring in migraine within relatively short periods of time. We hypothesized that the presence of an increased number of migraine attacks occurred between the repeated observations would be indicative of longitudinal migraine-related brain dysfunction.
We have shown previously that migraine has a greater influence in females and leads to more dysfunctional brain activity in their resting functional networks compared to males.4 To test our hypothesis, we focused on a group of female migraineurs without aura. Regional similarity and functional connectivity analysis were employed to investigate both the local site of the brain abnormalities and the entire dysfunctional network in migraine.
All research procedures were approved by the West China Hospital Subcommittee on Human Studies and were conducted in accordance with the Declaration of Helsinki. All subjects gave written, informed consent to participate after the experimental procedures had been fully explained and they were informed that they could stop participating at any time.
Nineteen right-handed PM without aura [all female, age 21.8±2.3 years (mean±SD), migraine duration of 9.1±2.6 years] who did not have any clinical affective disorder were recruited. Twenty, education- and gender-matched, healthy, righthanded healthy controls (HC; age 22.4±3.1 years) were recruited from the local community. The controls had experienced no headache during the previous year and had no family members who suffered regularly from a migraine or other headaches. All of the patients were screened in accordance with the International Headache Society criteria.8 The diagnostic criteria of the International Headache Society for migraine without aura include the occurrence of at least five headache attacks that fulfill the following criteria: 1) a unilateral and/or pulsating headache, 2) headache attacks lasting 4-72 hours (untreated or unsuccessfully treated), 3) presence of nausea and/or vomiting, photophobia, and phonophobia during the headache, 4) and the headache being disabling.9 The exclusion criteria were 1) macroscopic brain T2-visible lesions on magnetic resonance imaging (MRI) scans, 2) existence of a neurological disease, 3) pregnancy or menstrual period, 4) alcohol, nicotine, or drug abuse, or 5) claustrophobia.
All subjects submitted to a resting-state functional MRI (fMRI) scan at study entrance (i.e., baseline), and all of the patients underwent a second MRI scan within 6 weeks (39-45 days) using the same MR imager and acquisition protocol. For all of the patients, the scans were not performed within 72 hours before, during, or 24 hours after a migraine attack. The drugs used for the prophylaxis of migraine were stopped 6 weeks before the experiment; however, the subjects were allowed to take pirprofen when their headache was difficult to endure. Detailed information about the patients' drug intake was obtained. The Medication Quantification Scale was used to quantify the consumption of the drug at each visit, and the dosage and duration of the drug used are presented as scalar values. Patients were required to keep a headache diary to record their headache activity, including the migraine attack frequency, migraine attack duration, and average pain intensity of the headache (on a scale from 0 to 10 scale, with 10 being the most intense pain imaginable).
All subjects underwent resting-state fMRI scans using a 3-T MR system (GE EXCITE, Milwaukee, WI, USA) with an eight-channel phased-array head coil. Routine T2-weighted images [repetition time (TR)/echo time (TE)=4680.0 ms/105.2 ms, matrix size=256×256, field of view (FOV)=256×256 mm2, and section thickness=5 mm] and T1-weighted images (TR/TE=2360.4 ms/21.9 ms, matrix size=256×256, FOV=256×256 mm2, and section thickness=5 mm) were examined by an expert radiologist to exclude the possibility of clinically silent lesions. The fMRI images were obtained using an echoplanar imaging (EPI) sequence with the following parameters: 30 continuous slices with slice thickness=5 mm, TR=2000 ms, TE=30 ms, FOV=240×240 mm2, and data matrix=64×64. A total of 205 volumes was acquired for each subject, requiring a total scan time of 410 s. Subjects were instructed to rest with their eyes closed, not to think about anything in particular, and not to fall asleep.
The first five volumes were discarded to eliminate the non-equilibrium effects of magnetization and to allow subjects to acclimatize to the scanning environment. Image preprocessing was carried out using Statistical Parametric Mapping 5 software (http://www.fil.ion.ucl.ac.uk/spm). All data sets were processed using the following steps: 1) compensation of systematic slice-dependent time shifts, 2) elimination of systematic odd-even slice intensity differences due to the acquisition of interleaved images, 3) rigid-body correction for geometrical displacements caused by head movement, 4) spatial normalization with the Montreal Neurological Institute (MNI) EPI template image using an optimum 12-parameter affine and nonlinear cosine basis function transformation, resampled to 3-mm isotropic voxels, and 5) physiological and high-frequency noise correction by applying a band-pass filter from 0.01 to 0.1 Hz. No subjects exhibited head motions exceeding 1 mm of translation or 1 degree of rotation in any direction. There were no significant between-group differences in head motion (maximal translational and maximal rotational head deviation).
The regional homogeneity (ReHo) method was used to measure the intracluster information similarity, which might reflect the coherence of spontaneous neuronal activity.10 Recent studies found that ReHo can be a highly reliable (test-retest) measure of brain activity,11 and indicated that the method may serve as a valuable imaging feature for exploring pathological changes in brain function.10 By computing Kendall's coefficient of concordance12 for each voxel, the ReHo characterized the similarity of the time series of a given voxel to those of its nearest neighbors on a voxel-by-voxel basis.10 Individual ReHo maps were generated, with a Gaussian kernel with a full-width at half-maximum of 4 mm being used to smooth the images in order to reduce noise and residual differences.6 All of the ReHo analytical procedures were performed using the Resting-State fMRI Data Analysis Toolkit (X.-W. Song et al., Beijing Normal University, Beijing, China; http://www.restfmri.net). A two-sample t-test was used to compare the ReHo results between the PM and HC groups, and a paired t-test was used to compare changes in the ReHo values between the two resting scans in PM. The false discovery rate (FDR) was used to correct the multiple comparisons.
A network connectivity analysis was employed to assess the network properties in any abnormal brain regions. Building upon the patients' ReHo maps in the data sets, we investigated the distribution of the voxels that exhibited significant changes in ReHo values. The MNI coordinates of the maximally abnormal voxel within an anatomical area (minimum cluster size of 3 voxels) were chosen, and a sphere with a diameter of 12 mm was drawn around these coordinates. The voxels located in the white matter and ventricles within each sphere were removed to ensure the integrity of their structure and function. This process yielded several regions of interest (ROIs), which were considered as a set of nodes in the subsequent connectivity analysis. The mean time courses from the deep white matter, ventricles, and the six rigid-body motion parameters were regressed out from the fMRI time series of the selected ROIs. We computed the mean time series of each ROI and obtained the Pearson correlation coefficient matrix between all possible connections of the region pair within each individual patient, and all correlation values were converted using a Fisher Z-transformation. Nonparametric permutation tests were used to detect the presence of any significant group differences in the present study.13
The headache attack frequency was significantly higher at the 6-week follow-up examination (9.88±5.95) than at study entrance (4.76±3.27, p<0.05) (Table 1), whereas the pain intensity (5.05±1.1 and 5.6±0.9, respectively, p>0.05) (Table 1) and headache attack duration (5.09±3.18 and 7.95±6.6 years, respectively, p>0.05) (Table 1) did not increase over time.
Between-group differences (i.e., PM vs. HC) in brain local activity were found for the thalamus (THA), putamen (PUT), brainstem (BS), cingulate cortex, inferior parietal gyrus [Brodmann's area 40], hippocampus, orbitofrontal cortex (OFC), and the occipital cortex (p<0.05, FDR-corrected) (Fig. 1, Table 2). Moreover, the ReHo values for PM were significantly decreased in the PUT (MNI coordinate center 27, 6, 0), BS (-3, -27, -3), THA (12, -15, 12), temporal cortex, and cerebellum, and were significantly increased in the OFC (3, 33, -15) and secondary somatosensory cortex (SII, BA40; -54, -45, 39) relative to the baseline level (p<0.05, FDR-corrected) (Fig. 2). Significant correlations were found between changes in headache attack frequency and ReHo values in the OFC and SII (p<0.05), but not in the PUT, THA, and BS (Fig. 2).
To assess the functional connectivity among those local abnormalities, the PUT, BS, THA, OFC, and SII were chosen as the ROIs for the functional connectivity analysis (see Methods). As can be seen from Fig. 3, the changes in the functional connectivity intensity in the eight connections were significantly correlated with the changes in headache attack frequency (p<0.05) (Fig. 3). However, not all of the connections exhibited significant between-group differences. Five significantly increased connections were found on different resting scans (nonparametric permutation tests, p<0.01; red and blue lines in Fig. 3B). Analysis of the changes in the intensities of the PUT-THA and PUT-OFC connections revealed a positive correlation with the changes in headache attack frequency (red lines in Fig. 3B). The PUT-SII and PUT-BS connections were significantly negatively correlated with the changes in headache attack frequency (blue lines in Fig. 3B).
The main results of this study are as follows: 1) migraine disrupted the brain activity in several pain-processing brain regions, 2) the changes in ReHo values in these regions between repeated observations were significantly correlated with the changes in headache attack frequency, and 3) longitudinal alterations in connectivity characteristics converged at the PUT.
Abnormal ReHo value changes were found at the follow-up examination in the PUT, THA, BS, OFC, and SII. Several studies have found a network of brain regions (pain matrix) that are consistently activated in response to acute painful stimuli, and some of these brain areas also exhibit dysfunctional activities in chronic pain conditions.1,14 Our findings are consistent with those of previous studies. The prefrontal cortex, THA, basal ganglia, parts of the BS, and the somatosensory cortex are the main regions that process and regulate pain information.15,16 ReHo measures the similarity of low-frequency fluctuations within a local brain area, which may reflect the coherence of neuronal activity.10,17 Hence, abnormal ReHo values indicate that resting neural function in the aforementioned brain regions was abnormally synchronized in PM between the two different observation points, which may reflect differences in the pain-related information processing induced by the increased headache activity. The significant correlation between the changes in headache attack frequency and ReHo values may support our assumption. Several studies have shown that chronic headache attack can be understood not only as a dysfunctional perceptual state but also as a result of brain plasticity in response to long-term chronic pain.18 Hence, these brain abnormalities may be the result of aberrant development of the brain in the presence of ongoing central changes, rather than as an adventitious insult or lesion to a normal brain. This process may be maladaptive and directly related to the worsening of headache activity in migraine. The current results suggest a relationship between progressive brain functional alteration and increased migraine attack frequency in patients. However, whether these longitudinal ReHo changes can be employed to predict progressive neuronal changes or to evaluate the worsening of clinical symptoms in particular patients requires further investigation.
The BS is the main station for integrating the cognitive and autonomic processing of nociceptive information,19 and is considered to be made dysfunctional by migraine pathophysiology.20,21 Positron-emission tomography has highlighted neuronal activation in the BS in PM.22,23 Similar neuronal changes were found in experimental animals during trigeminovascular activation.24,25 Moreover, measurement of the neural activity before and after successful treatment in migraine revealed that the BS remained activated,26,27 indicating that the BS might have generated the migraine attack.14,19 The findings of the present study cannot be used to determine the cause-and-effect relationship between BS modulation and migraine symptoms. The longitudinal brain changes in the BS were independent of changes in the attack frequency. We assumed that changes or dysfunction in the BS may present a migraine-related brain state, creating a referred headache, which caused the corresponding dysfunction of the brain regions involved in the pain processing networks.
Another important finding of this study was the identification of the disproportionately dysfunctional connectivity in the PUT, while acting as a critical intermediate station for information processing among the selected ROIs in migraine. Several brain imaging studies have found abnormal activation in the basal ganglia, and notably in the PUT,14 in migraine. Moreover, the gray matter in the basal ganglia was altered in migraineurs with a higher attack frequency and longer duration of disease.7 One recent study found significantly lower activity in the PUT for the response to painful heat stimulation in high frequency episodic migraine as compared to low frequency episodic migraine.3 That finding may support the inference that the dysfunction in the PUT would be aggravated by an increasing migraine frequency. Our results are consistent with those obtained from previous studies. We found that the PUT serves to integrate diverse information among other migrainerelated brain regions, and we infer from this that preventative treatments that are designed to target the dysfunction of the PUT in migraine may slow the disease process and relieve pain in migraineurs.
Two issues raised by the present study need to be addressed further. First, little is known about the differences between progressive patients and nonprogressive patients with respect to dysfunctional brain organization in migraine pathophysiology. Second, the interregional functional connectivity analysis was not corrected for in our multiple comparisons. The complex patterns of negative and positive correlations between functional connectivity and migraine attack frequency were difficult to explain; these results should thus be interpreted with caution.
In summary, a sustained increase in headache attack frequency and longitudinal functional brain activity changes were found to occur in migraineurs within a relatively short period of time. These findings may reflect brain alteration dynamics resulting from a developed headache. If these results can be replicated in a large sample, it may help us to better understand the progression of migraine and improve our ability to implement effective treatments.
Figures and Tables
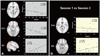 | Fig. 2Longitudinal brain changes in regional homogeneity (ReHo) occurred in migraine patients (PM) within a relatively short period of time (p<0.01, corrected for the false discovery rate). Warm colors indicate ReHo increases in PM relative to the baseline level. BS: brainstem, OFC: orbitofrontal cortex, PUT: putamen, SII: secondary somatosensory A B cortex, THA: thalamus. |
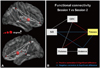 | Fig. 3Abnormal functional connectivity and its relationship with changes in headache attack frequency. The putamen (PUT), orbitofrontal cortex (OFC), secondary somatosensory cortex (SII), brainstem (BS), and thalamus (THA) were chosen as the regions of interest for the functional connectivity analysis (red balls). A: The gray lines indicate a significant correlation between the changes in attack frequency and the ratio of changes in functional connectivity intensity (p<0.05). B: The color connections indicate the significant changes in functional connectivity intensity in the migraine group between the repeated observations, and represent significant correlations between the changes in attack frequency (red lines: positive correlations; blue A B lines: negative correlations). |
Acknowledgements
This work was supported by the Project for the National Key Basic Research and Development Program (973) under Grant nos. 2012CB518501, 2014CB543203, and 2011CB707700; the National Natural Science Foundation of China under Grant nos. 81227901, 81271644, 30930112, 81000640, 81000641, 81101036, 81101108, 81030027, 81301281, 31200837, and 31271063; the Natural Science Basic Research Plan in Shaanxi Province of China under Grant no. 2014JQ4118; and the Fundamental Research Funds for the Central Universities.
References
1. May A. New insights into headache: an update on functional and structural imaging findings. Nat Rev Neurol. 2009; 5:199–209.

3. Maleki N, Becerra L, Pendse G, Brawn J, Bigal M, et al. Migraine attacks the Basal Ganglia. Mol Pain. 2011; 7:71.

4. Liu J, Qin W, Nan J, Li J, Yuan K, Zhao L, et al. Gender-Related Differences in the Dysfunctional Resting Networks of Migraine Suffers. PLoS One. 2011; 6:e27049.

5. Liu J, Zhao L, Li G, Xiong S, Nan J, Li J, et al. Hierarchical Alteration of Brain Structural and Functional Networks in Female Migraine Sufferers. PloS One. 2012; 7:e51250.

6. Yu D, Yuan K, Zhao L, Zhao L, Dong M, Liu P, et al. Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR Biomed. 2012; 25:806–812.

7. Schmitz N, Admiraal F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008; 48:1044–1055.

8. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004; 24:Suppl 1. 9–160.
9. Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006; 296:1274–1283.

10. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004; 22:394–400.

11. Zuo XN, Xu T, Jiang L, Yang Z, Cao XY, He Y, et al. Toward reliable characterization of functional homogeneity in the human brain: Preprocessing, scan duration, imaging resolution and computational space. Neuroimage. 2013; 65:374–386.

12. Kendall MG, Gibbons JD. Rank correlation methods. 5th ed. New York: Oxford University Press;1990.
13. Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999; 18:32–42.

15. Davis KD, Moayedi M. Central Mechanisms of Pain Revealed Through Functional and Structural MRI. J Neuroimmune Pharmacol. 2013; 8:518–534.

17. Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, et al. Regional homogeneity changes in patient's with Parkinsons disease. Hum Brain Mapp. 2009; 30:1502–1510.

18. Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS One. 2011; 6:e26010.

19. Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci. 2011; 12:570–584.

20. Goadsby PJ, Lipton RB, Ferrari MD. Migraine-current understanding and treatment. N Engl J Med. 2002; 346:257–270.
21. May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab. 1999; 19:115–127.

22. Weiller C, Mav A, LlMmroth V, Jüptner M, Kaube H, Schayck RV, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995; 1:658–660.

23. Afridi SK, Giffin NJ, Kaube H, Friston KJ, Ward NS, Frackowiak RS, et al. A positron emission tomographic study in spontaneous migraine. Arch Neurol. 2005; 62:1270–1275.

24. Tassorelli C, Joseph SA. Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res. 1995; 682:167–181.

25. Lambert GA, Donaldson C, Boers PM, Zagami AS. Activation of trigeminovascular neurons by glyceryl trinitrate1. Brain Res. 2000; 887:203–210.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download