Abstract
Background and Purpose
Cerebral microbleeds (CMBs) are associated with various pathologies of the cerebral small vessels according to their distribution (i.e., cerebral amyloid angiopathy or hypertensive angiopathy). We investigated the association between CMB location and kidney function in acute ischemic stroke patients.
Methods
We enrolled 1669 consecutive patients with acute ischemic stroke who underwent gradient-recalled echo brain magnetic resonance imaging. Kidney function was determined using the estimated glomerular filtration rate (eGFR). CMBs were classified into strictly lobar, strictly nonlobar (i.e., only deep or infratentorial), and a combination of both lobar and nonlobar. Multinomial logistic regression analyses were used to determine the factors associated with the existence of CMBs according to their location.
Results
The patients were aged 66±12 years (mean±standard deviation), and 61.9% (1033/1669) of them were male. CMBs were found in 27.0% (452/1669) of the patients. The stroke subtypes of small-artery occlusion and cardioembolism occurred more frequently in those with strictly nonlobar CMBs (10.8%) and strictly lobar CMBs (48.8%), respectively. The mean eGFR was lower in the strictly nonlobar CMBs group (72±28 mL/min/1.73 m2) and the both lobar and nonlobar CMBs group (72±25 mL/min/1.73 m2) than in the no-CMBs group (86±29 mL/min/1.73 m2). Multivariate multinomial logistic regression revealed that eGFR <60 mL/min/1.73 m2 was independently related to strictly nonlobar CMBs (odds ratio=2.63, p=0.001).
Cerebral microbleeds (CMBs) are tiny perivascular hemorrhages characterized by the presence of hemosiderin-laden macrophages close to disrupted cerebral small vessels, which appear as small and rounded lesions of low signal intensity on gradient-recalled echo (GRE) magnetic resonance imaging (MRI).1,2 The location of CMBs varies according to the vascular pathophysiology: deep or infratentorial (nonlobar) CMBs are related to angiopathy with classical vascular risk factors, and lobar CMBs are related to cerebral amyloid angiopathy (CAA).1
Impaired kidney function is an independent risk factor for cardiovascular disease3 and stroke.4 Since the kidney and brain contain similar low-resistance microvascular structures and as end organs they both receive blood at a continuously high flow rate, they are vulnerable to blood pressure fluctuations.5 It is therefore possible that impaired kidney function is associated with pathologies involving small vessels (end arteries) in the brain.6 In support of this theory, it was found that impaired kidney function was a significant risk factor for CMBs in stroke.7,8,9 However, the relationship between location of CMBs and impaired kidney function is not well known.
Given that the mechanisms underlying the evolution of CMBs differ between nonlobar and lobar lesions, the association between CMBs and impaired kidney function may differ according to their distribution. Therefore, we investigated the relationship between CMBs and impaired kidney function, and explored whether that association varies according to the location of CMBs in acute ischemic stroke patients.
We enrolled 1902 consecutive patients between January 2009 and December 2011 with acute cerebral infarction who were admitted within 7 days after symptom onset.10 All patients were evaluated based on the standard protocol of our hospital, which includes brain imaging studies [computed tomography (CT) and/or MRI], vascular imaging studies (digital subtraction angiography, MR angiography, or CT angiography), chest radiography, 12-lead electrocardiography, routine blood tests, and continuous electrocardiographic monitoring during their stay in the stroke unit. Brain MRI was performed within 3 days after admission. The estimated glomerular filtration rate (eGFR), which reflects kidney function, was calculated using the Modification of Diet in Renal Disease formula as11 eGFR=186.3×(serum creatinine-1.154)×(age-0.203)×(0.742 for women). Serum creatinine was measured at admission. If the eGFR was less than 90 mL/min/1.73 m2, serum creatinine was checked at least twice, and the value obtained closest to the time at which brain MRI was performed was used for this study. The stroke subtype was determined based on the Trial of Org 10172 in Acute Stroke Treatment classification system.12 Patients were excluded from this study if they did not undergo brain MRI due to patient refusal, claustrophobia, or the presence of metallic material in the body (n=107). Patients with stroke due to other determined etiology (rare causes, n=42), unavailable GRE images (n=45), lack of any blood laboratory results used for this study (n=16), poor image quality (n=15), or lack of vascular imaging studies (n=8) were also excluded. Ultimately, 1669 patients were included in this study, which was approved by the Severance Hospital Institutional Review Board of the Yonsei University Health System.
Hypertension was defined as being present when a patient had been taking blood-pressure-lowering agents or had a resting systolic/diastolic blood pressure of ≥140/≥90 mm Hg on repeated measurements. Diabetes mellitus was diagnosed when the patient had a fasting blood glucose level of ≥7.0 mmol/L or was being treated with oral glucose-lowering medications or insulin. Hyperlipidemia was diagnosed if the patient had total cholesterol and low-density lipoprotein levels of ≥6.2 and ≥4.1 mmol/L, respectively, or if the patient had taken lipid-lowering medications after a diagnosis of hyperlipidemia. Patients were defined as smokers if they were current smokers or had stopped smoking within 1 year before the index stroke. Body mass index was calculated by dividing weight (in kilograms) by height (in meters) squared. Coronary artery disease was defined as myocardial infarction, unstable angina, or angiographically confirmed coronary artery occlusive disease.
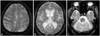
All MRI examinations were performed using a 3.0-T MRI system (Achieva 3.0T, Philips Medical Systems, Best, The Netherlands; or MAGNETOM Trio 3.0T, Siemens, Germany). Brain MR images were obtained parallel to the orbitomeatal line using the following parameters, according to a previous study:13 fluid-attenuated inversion recovery (FLAIR) images-repetition time (TR)/echo time (TE)=9000 ms/120 ms, field of view=230×230 mm2, pixel spacing=0.449 mm/0.449 mm, and slice thickness=5 mm; T2-weighted images-TR/TE=9000 ms/100 ms, field of view=230×230 mm2, pixel spacing=0.240 mm/0.240 mm, and slice thickness=5 mm; and GRE images-TR/TE=600 ms/16 ms, field of view=250×250 mm2, pixel spacing=0.449 mm/0.449 mm, and slice thickness=5 mm. CMBs were defined as small, round, and hypointense lesions with a longest diameter in the range 3-10 mm. They were classified as either strictly lobar, strictly nonlobar (i.e., only in the deep or infratentorial areas), and a combination of both lobar and nonlobar on GRE images (Fig. 1). Strictly lobar CMBs were categorized into possible or probable CAA using previously reported methods.14,15 The presence of CMBs on GRE was independently reviewed by two neurologists (T.-J.S. and J.K.) who were blinded to the clinical information. The interobserver agreement regarding the existence of CMBs was excellent (κ=0.92). Asymptomatic lacunar infarction was defined as a hyperintense lesion of ≥3 mm and ≤15 mm on T2-weighted images, and without a relevant history of symptoms or signs. We also investigated the severity of leukoaraiosis (LA) in our study patients. The extent of LA was determined based on FLAIR images of the periventricular white matter (PVWM) or deep white matter (DWM) using a previously established method.16 LA in PVWM was classified as follows: grade 0, absent; grade 1, caps or pencil-thin lining; grade 2, smooth halo; and grade 3, irregular PVWM extending into DWM. LA in DWM was categorized as follows: grade 0, absent; grade 1, punctate foci; grade 2, beginning confluence of foci; and grade 3, large confluent areas. For statistical analyses, LA was defined as low-grade (Fazekas' score of 0 or 1 in PVWM and DWM) and high-grade (Fazekas' score of ≥2 in PVWM or DWM) white-matter change.13
Statistical analyses were performed using the Windows SPSS software package version 18.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are reported as mean±standard deviation values or as odds ratios and 95% confidence intervals. Categorical variables are presented as frequencies (i.e., n) and percentages. For comparison, patients were grouped according to their eGFR values as ≥90, 60-90, and <60 mL/min/1.73 m2. One-way analysis of variance with Bonferroni corrected post-hoc analyses were used to compare continuous values according to CMB location. Categorical variables were compared by using the chi-square test or Fisher's exact test. The association between number of CMBs and eGFR among the different CMB types was explored using Spearman correlation analysis. Multinomial uni- and multivariate logistic regression analyses were conducted using the no-CMB group as the reference group for determining factors related to the existence of CMBs according to their location. Age, sex, and variables with p<0.1 in univariate analyses were entered into multivariate analyses. A two-tailed p value of <0.05 was considered significant.
The demographic data of the study population are given in Table 1. The age was 66±12 years and 61.9% (1033/1669) of the study participants were male. CMBs were found in 27.0% of the subjects (452/1669; strictly lobar in 80 patients, strictly nonlobar in 278 patients, and a combination of both lobar and nonlobar in 94 patients). Of the 80 patients with strictly lobar CMBs, 15 (18.7%) had probable CAA and 65 (81.3%) had possible CAA. Patients with CMBs were older than those without CMBs. A history of hypertension, stroke, coronary artery disease, and antiplatelet medication, the presence of an asymptomatic lacunar infarction, and high-grade white-matter changes were more frequent in the three groups with CMBs than in the no-CMBs group (Table 1). Smoking was more frequent in the no-CMBs group than in the three groups with CMBs. The eGFR was lower in the strictly nonlobar CMBs group (72±28 mL/min/1.73 m2, p=0.001) and the both lobar and nonlobar CMBs group (72±25 mL/min/1.73 m2, p=0.001) than in the no-CMBs group (86±29 mL/min/1.73 m2). After categorizing the patients according to their eGFR values, patients with eGFR <60 mL/min/1.73 m2 were more common in the strictly nonlobar CMBs group and the both lobar and nonlobar CMBs group (p=0.001). The number of CMBs was inversely correlated with the presence of strictly nonlobar CMBs (r=-0.123, p=0.001) and both lobar and nonlobar CMBs (r=-0.182, p=0.001), but was not correlated with strictly lobar CMBs (r=-0.041, p=0.142).
In multivariate multinomial logistic regression, after adjusting age, sex, and p<0.1 in univariate analysis, a history of hypertension and coronary artery disease and an eGFR of <60 mL/min/1.73 m2 remained significant for strictly nonlobar CMBs (e.g., odds ratio=2.63, p=0.001 for an eGFR of <60 mL/min/1.73 m2) (Table 2). When comparing the association between stroke subtypes and CMB location using multivariate logistic regression with the subtype of undetermined etiology due to a negative evaluation as a reference, cardioembolism was more frequent in the strictly lobar CMBs group but less frequent in the strictly nonlobar CMBs group (Table 2). In addition, asymptomatic lacunar infarction and high-grade white-matter changes were independently related with CMBs, regardless of their location. We further analyzed the association between CMBs and impaired kidney function according to the presence or absence of diabetes mellitus, because a previous study found that impaired kidney function was associated with CMBs only among those without diabetes mellitus.7 The association between lower eGFR (<60 mL/min/1.73 m2) and strictly nonlobar CMBs was significant in patients without (Supplementary Table 1) but not with (Supplementary Table 2) diabetes mellitus.
Our findings demonstrate that the association between CMBs and impaired kidney function differs according to the location of the CMBs. Moreover, a low eGFR was correlated with strictly nonlobar CMBs but not with lobar CMBs, which suggests that the associations between CMBs and impaired kidney function noted in previous studies may have referred to strictly nonlobar CMBs rather than CMBs in other locations.
While CMBs have similar pathologies as putative markers of bleeding-prone angiopathy, they can develop via two distinct mechanisms: small-vessel-disease-related angiopathy due to classical vascular risk factors, or CAA. Preferential sites for CMBs that arise due to classical vascular risk factors and CAA are different because small-vessel pathologies develop in deep brain areas where the perforating arteries provide the blood supply, whereas CAA develops mainly in the lobar area. CAA affects the arteries of the leptomeninges and cortex, and essentially does not involve the deep perforating arteries at the base of the brain and brain stem.17 In addition, a recent amyloid positron-emission tomography study showed that amyloid burden was correlated only with lobar CMBs.18 Moreover, the risk factors for CAA are known to be associated with the apolipoprotein e-4 allele but not with kidney dysfunction.14 On the contrary, hypertensive vasculopathy most severely affects the perforating arteries of the brain.19 In this regard, the predilection site of CMBs may vary according to the pathophysiological mechanism of the angiopathy. The results of the present study support these previous findings.
Furthermore, impaired kidney function is associated with cerebral small-vessel disease, independent of other classical vascular risk factors.6,20 The association between impaired kidney function and small-vessel pathologies in strictly nonlobar areas may be ascribed to the anatomical similarities between the vessels and the mechanism underlying dysfunction of the endothelial and smooth-muscle cells.21 In the kidney, small juxtamedullary afferent arterioles are subjected to high pressure and maintain a strong vascular tone.22 Since the perforating arteries in the brain share similar vascular components to the kidney,22 the continuous delivery of high pressure (as in hypertension) to the deep perforating arteries in the brain may damage those arteries, resulting in the small-vessel pathologies that produce CMBs. Impaired kidney function is also associated with endothelial dysfunctions such as reduced nitric oxide secretion,23 increased oxidative stress,24 and up-regulation of cellular adhesion molecules,23 which are also involved in cerebral small-vessel pathologies.25 Therefore, the anatomical and pathophysiological similarities of deep perforating-vessel damage in the kidney and brain may explain the association between impaired kidney function and the strictly nonlobar location of CMBs.
Another finding of our study was that CMBs were related to asymptomatic lacunar infarction and high-grade white-matter changes, regardless of the location of CMBs, while impaired kidney function was associated with only strictly nonlobar CMBs. Although hypertensive vasculopathy is the main mechanism underlying small-vessel disease in the deep perforating artery territory, a chronic hypertensive state can also induce autoregulatory dysfunction of the pial superficial perforating arteries, which may result in damage to the smooth-muscle layer and microhemorrhage.26,27 Moreover, a previous study found that the volume of white-matter changes and counts of asymptomatic lacunar infarctions were positively associated with the existence of CMBs, independent of their distribution.18 However, it is uncertain why the association between CMBs and their location in impaired kidney function differed from that in asymptomatic lacunar infarction or high-grade white-matter changes in our study population.
In our study, coronary artery disease was associated with strictly nonlobar CMBs. This finding could be attributed to the risk factors of coronary artery disease being similar to those of deep or infratentorial CMBs, such as hypertension, which is the main causative factor of nonlobar CMBs. Among the stroke subtypes, cardioembolism was positively associated with strictly lobar CMBs, but negatively associated with strictly nonlobar CMBs. The negative association between strictly nonlobar CMBs and cardioembolism may be due to the frequency of hypertension being lower in cardioembolism (71.1%) than in other stroke subtypes: large-artery atherosclerosis, 76.5%; small-artery occlusion, 83.0%; undetermined etiology due to negative evaluation, 73.2%; and undetermined etiology due to two or more causes identified, 75.1%. However, the reason why cardioembolism was associated with strictly lobar CMBs in our study population remains to be elucidated.
This study was subject to two main limitations. First, markers of kidney dysfunction other than eGRF, such as microalbuminuria, were not evaluated.28 However, eGRF is a well-known and valid marker of renal dysfunction.11 Second, our explanation regarding the mechanism underlying the differential association between kidney dysfunction and CMB distribution is based on the assumption that the former is related to vascular-risk-factor-related angiopathy and the latter is related to CAA; however, these diagnoses could not be pathologically confirmed in our study.
In conclusion, impaired kidney dysfunction was independently associated with strictly nonlobar CMBs but not with lobar CMBs. Our findings also indicate that CMBs should be considered relative to their distribution when evaluating their relationships or prognoses.
Figures and Tables
Fig. 1
Representative images demonstrating the classification of cerebral microbleeds into (A) strictly lobar CMBs, (B) strictly nonlobar CMBs, and (C) both lobar and nonlobar CMBs. CMBs: cerebral microbleeds.
Acknowledgements
This work was supported by a grant from the Korea Healthcare Technology Research and Development Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea (HI10C2020).
References
1. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009; 8:165–174.

2. Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999; 20:637–642.
3. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351:1296–1305.

4. Nakayama M, Metoki H, Terawaki H, Ohkubo T, Kikuya M, Sato T, et al. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population--the Ohasama study. Nephrol Dial Transplant. 2007; 22:1910–1915.

5. O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005; 46:200–204.
6. Khatri M, Wright CB, Nickolas TL, Yoshita M, Paik MC, Kranwinkel G, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS). Stroke. 2007; 38:3121–3126.

7. Ryu WS, Lee SH, Kim CK, Kim BJ, Yoon BW. The relation between chronic kidney disease and cerebral microbleeds: difference between patients with and without diabetes. Int J Stroke. 2012; 7:551–557.

8. Cho AH, Lee SB, Han SJ, Shon YM, Yang DW, Kim BS. Impaired kidney function and cerebral microbleeds in patients with acute ischemic stroke. Neurology. 2009; 73:1645–1648.

9. Shima H, Ishimura E, Naganuma T, Yamazaki T, Kobayashi I, Shidara K, et al. Cerebral microbleeds in predialysis patients with chronic kidney disease. Nephrol Dial Transplant. 2010; 25:1554–1559.

10. Lee BI, Nam HS, Heo JH, Kim DI. Yonsei Stroke Team. Yonsei Stroke Registry. Analysis of 1,000 patients with acute cerebral infarctions. Cerebrovasc Dis. 2001; 12:145–151.
11. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999; 130:461–470.

12. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.

13. Song TJ, Kim J, Lee HS, Nam CM, Nam HS, Heo JH, et al. The frequency of cerebral microbleeds increases with CHADS(2) scores in stroke patients with non-valvular atrial fibrillation. Eur J Neurol. 2013; 20:502–508.

14. Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008; 70:1208–1214.

15. Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001; 56:537–539.

16. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalites at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987; 149:351–356.
17. Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol. 2011; 7:1–9.

18. Park JH, Seo SW, Kim C, Kim GH, Noh HJ, Kim ST, et al. Pathogenesis of cerebral microbleeds: In vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann Neurol. 2013; 73:584–593.

20. Takahashi W, Tsukamoto Y, Takizawa S, Kawada S, Takagi S. Relationship between chronic kidney disease and white matter hyperintensities on magnetic resonance imaging. J Stroke Cerebrovasc Dis. 2012; 21:18–23.

21. Iadecola C, Pelligrino DA, Moskowitz MA, Lassen NA. Nitric oxide synthase inhibition and cerebrovascular regulation. J Cereb Blood Flow Metab. 1994; 14:175–192.

22. Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res. 2009; 32:115–121.

24. Annuk M, Zilmer M, Lind L, Linde T, Fellström B. Oxidative stress and endothelial function in chronic renal failure. J Am Soc Nephrol. 2001; 12:2747–2752.

25. Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian Stroke Prevention Study. Stroke. 2005; 36:1410–1414.

26. Román GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002; 1:426–436.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download