Abstract
Background
Infarct of the anterior spinal artery is the most common subtype of spinal cord infarct, and is characterized by bilateral motor deficits with spinothalamic sensory deficits. We experienced a case with atypical anterior-spinal-artery infarct that presented with bilateral hand weakness but without sensory deficits.
Case Report
A 29-year-old man presented with sudden neck pain and bilateral weakness of the hands. Magnetic resonance imaging (MRI) of the brain did not reveal any lesion. His motor symptoms improved rapidly except for mild weakness in his left wrist and fingers. Magnetic resonance angiography showed proximal occlusion of the left vertebral artery; a spine MRI revealed left cervical cord infarction.
Spinal cord infarction is much less frequent than cerebral infarction, accounting for only 1% of all strokes.1 The anterior spinal artery supplies the anterior two-thirds of the spinal cord via the sulcal (central) artery. This usually results in anterior spinal artery infarcts presenting with profound bilateral motor deficits, sensory disturbances, and spinothalamic sensory deficits.2,3 Although the mechanisms are not completely understood, there are some case reports of restrictive clinical syndromes, such as the 'man-in-the-barrel syndrome' and the sulcal artery syndrome.4,5 Here we describe a patient who presented with bilateral hand weakness but without sensory deficits due to left vertebral artery occlusion.
A healthy 29-year-old man without any vascular risk factors presented with sudden neck pain and bilateral hand weakness. After flexing his neck while tying his shoelaces, the patient experienced a sudden, severe pain (with a maximum score on a visual analog scale) that started in the posterior neck and spread rapidly across the entire head. Bilateral hand weakness that followed the pain onset prevented the patient from tying his shoelaces. The patient's vital signs were stable upon hospital admission, except for markedly elevated blood pressure (202/108 mm Hg). The initial neurological examination revealed weakness in both flexion and extension of the wrists and fingers bilaterally, and during left leg extension [Medical Research Council (MRC) grade 4]. However, the deep tendon reflexes were normal and the patient denied hypoesthesia in any sensory modality including pain, temperature, proprioception, and vibration. A detailed examination did not reveal any cortical sensory sign such as graphesthesia. The Romberg test produced a negative result. Laboratory findings were normal, and chest radiography did not reveal any pathology; an electrocardiogram also produced no evidence of ischemia or arrhythmia. Furthermore, 2-D echo and transesophageal echocardiograms provided no evidence for an embolic stroke. Subsequent 24-hour Holter monitoring failed to identify any arrhythmia or atrial fibrillation. His motor symptoms improved rapidly within 1 day except for mild weakness of his left wrist (MRC grade of flexion/extension IV/IV) and fingers (MRC grade of flexion/extension IV/IV; slightly more severe in the 5th finger). Brain computed tomography (CT) and brain magnetic resonance imaging (MRI) did not reveal any signal abnormalities or mass lesions. However, CT aortography identified severe stenosis and occlusion of the left vertebral artery, and a pseudo lumen with mural thrombi was suspected on cervical MRI (Fig. 1). These clinical and radiological findings implicated arterial dissection. MRI of the cervical segment produced high signal intensities in the left gray matter of spinal cord at the C3, C4, and C6 levels on diffusion-weighted images (Fig. 2). The patient was discharged on an oral anticoagulant. Magnetic resonance angiography performed 3 months after symptom onset showed persistent occlusion of the left vertebral artery.
Unique features of this spinal-cord-infarct patient were bilateral hand weakness, absence of sensory deficits, and rapid improvement of motor symptoms in the right hand and left leg.
Weakness of both arms has been reported previously in association with cervical cord infarcts, typically also with spinothalamic sensory deficit and vertebral artery occlusion.4,5,6,7 The mechanism that preserves leg strength in these cases is most likely collateral flow from the surrounding pial plexus.4 Nevertheless, weakness of both hands without sensory deficits is rare in infarcts of the cervical spinal cord, and its mechanism remains to be determined.7,8
The anterior spinal artery forms at the level of the foramen magnum from the branches of the vertebral artery, and gives rise to the sulcal (central) arteries that penetrate the right and left sides of the spinal cord.3 Unlike circumferential areas of the spinal cord, its interior has no anastomosis and the sulcal arteries are essentially end arteries. Therefore, the weakness of both hands in the present patient suggests that the end-arteries zone or the zone bordering the sulcal artery and circumferential artery corresponds to a region where hand motor neurons are located bilaterally.8 This would include the ventrolateral to lateral regions within the gray matter of the lower cervical spinal cord (Fig. 3).9,10 Alternatively, the hand motor areas may be more vulnerable than the more proximal arm motor areas. The hand motor area occupies a relatively large proportion of the cortical homunculus, so the hand motor area may occupy a larger area in the spinal gray matter, or require a larger blood supply.11,12
Finally, the isolated unilateral hand weakness of this patient may be related to variation in the branches of the sulcal artery. Successive sulcal arteries generally alternate in their distribution to the left or right side of the spinal cord, but not both; this would make unilateral involvement or improvement possible.5 Sensory deficit was absent in the present case. All subtypes of spinal cord infarcts are presumed to have sensory deficits, and therefore a pure motor presentation can result in misdiagnosis.2,3 Anterolateral involvement could explain the present atypical presentation, and bilateral presentation without facial weakness may indicate a spinal cord infarct.5
Transient ischemic attacks in the spinal cord reported occur primarily in the cervical cord, and recovery depends on the severity of the initial involvement and its collateral flow.1,2,6 Cervical lesions are generally less vulnerable to ischemic insults than are thoracic or lumbar lesions due to the intensive vascularization from the vertebral and radicular arteries.3,6,13 The rapid improvement in the present case might therefore have resulted from initial distal involvement and collateral flow from the intact right vertebral artery.
In conclusion, transient bilateral hand weakness can be the initial symptom of a spinal cord infarct, and unilateral hand weakness can be the sole symptom of a cervical cord infarct.
Figures and Tables
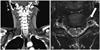 | Fig. 1A: Severe stenosis and occlusion of the proximal part of the left vertebral artery in a neck CT angiogram. B: Axial T2-weighted image showing severe stenosis of the left vertebral artery due to dissection, showing a pseudo lumen with mural thrombi (arrow), and a high signal intensity in the left gray matter of spinal cord at the C4 level. |
References
1. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006; 63:1113–1120.
2. Kumral E, Polat F, Güllüoglu H, Uzunköprü C, Tuncel R, Alpaydin S. Spinal ischaemic stroke: clinical and radiological findings and short-term outcome. Eur J Neurol. 2011; 18:232–239.

4. Berg D, Müllges W, Koltzenburg M, Bendszus M, Reiners K. Man-in-the-barrel syndrome caused by cervical spinal cord infarction. Acta Neurol Scand. 1998; 97:417–419.
5. Li Y, Jenny D, Bemporad JA, Liew CJ, Castaldo J. Sulcal artery syndrome after vertebral artery dissection. J Stroke Cerebrovasc Dis. 2010; 19:333–335.

6. Machnowska M, Moien-Afshari F, Voll C, Wiebe S. Partial anterior cervical cord infarction following vertebral artery dissection. Can J Neurol Sci. 2008; 35:674–677.

7. Park SW, Sohn SI, Cho YW, Lee H, Lim JG, Yi SD. Spinal cord infarction with anterior chest pain. J Korean Neurol Assoc. 2005; 23:840–841.
8. Pullicino P. Bilateral distal upper limb amyotrophy and watershed infarcts from vertebral dissection. Stroke. 1994; 25:1870–1872.

9. Parent A. Spinal cord: regional anatomy and internal structure. In : Parent A, editor. Carpenter's Human Neuroanatomy. 9th ed. Baltimore: Williams & Wilkins;1996. p. 325–367.
10. Alblas CL, Bouvy WH, Lycklama À Nijeholt GJ, Boiten J. Acute spinal-cord ischemia: evolution of MRI findings. J Clin Neurol. 2012; 8:218–223.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download