Abstract
Background and Purpose
Vascular shear stress is essential for maintaining the morphology and function of endothelial cells. We hypothesized that shear stress in the internal carotid artery (ICA) may differ between patients with ischemic stroke and healthy control subjects.
Methods
ICA shear stress was calculated in 143 controls and 122 patients with ischemic stroke who had a normal ICA or an ICA with <50% stenosis. The stroke group included patients who presented with a first-ever or recurrent ischemic stroke but excluded cardioembolic stroke and uncertain etiologies. Of the 122 patients, 107 (87.7%) and 15 (12.3%) patients were categorized as first-ever and recurrent stroke, respectively.
Results
Carotid diameters were significantly larger, and both peak-systolic and end-diastolic velocities were significantly lower in patients with ischemic stroke than in controls (all p values <0.05). Mean values of peak-systolic and end-diastolic shear stress in both ICAs were significantly lower in patients with ischemic stroke in models that adjusted for age, sex, and vascular risk factors (p for trend <0.05). The ICA shear stress was lowest in patients with recurrent stroke or the subtype of small-vessel occlusion. Higher peak-systolic and end-diastolic shear stresses in both ICAs were independently and negatively associated with ischemic stroke after adjusting for potential confounders (all p values <0.05).
Vascular shear stress contributes to the site specificity of atherosclerotic lesions; however, the utility of arterial shear stress has been limited due to the technical difficulty of measuring this stress and uncertainty about the implications of the mechanism for therapy. It is still unclear whether the ability to measure the arterial shear stress would improve diagnoses of vascular events. Improving the understanding of the pathophysiological role of vascular shear stress requires more investigations involving various methods and study designs.
The vascular-wall shear stress is the frictional force that acts tangentially. Vascular shear stresses within normal physiologic ranges are essential for maintaining endothelial-cell morphology (i.e., an elliptical shape with normal tight junctions) and function associated with the physiological release of nitric oxide.1 A low wall shear stress (<4 dyne/cm2) allows for active interactions between the cellular and plasmatic components of blood and the vessel walls,2 and potentially result in atherothrombosis through complex molecular and biomechanical mechanisms.3,4
The extracranial carotid artery was previously evaluated in calculations of arterial geometry-specific shear stress in patients with vascular events.5 Other studies have investigated shear stress in the carotid artery in association with atherothrombosis that caused ischemic stroke,6 and cardiovascular risk factors.7 A previous study found an association between shear stress in the common carotid artery (CCA) and ischemic stroke, after measuring the patient's blood viscosity.8
The carotid artery is divided into three segments: the CCA including the bulb, the internal carotid artery (ICA), and the external carotid artery. The ICA is a direct conduit for blood supply to cerebral tissues, and it exhibits a low resistance for cerebral autoregulation. The ICA shear stress is influenced by the cerebral blood flow, and may be more closely associated with stroke than are the other carotid segments.9 Stroke has a heterogeneous etiology, such as large-artery atherosclerosis (LAA) and small-vessel occlusion (SVO).10 A more comprehensive vascular imaging approach may be needed to understand the etiologic heterogeneity of stroke.
We hypothesized that ICA shear stress differs between patients with ischemic stroke and control subjects, and that it can provide discriminative information on the cerebral hemodynamics of ischemic stroke. To test this, we calculated the carotid artery shear stress along the carotid segments using duplex ultrasonography, and examined the association with ischemic stroke while considering potential confounders.
Consecutive patients referred to the Neurovascular Ultrasound Laboratory of the Stroke Centre of Chonbuk National University Hospital from January 2007 to March 2008 were included in this study. Neurosonologic examinations were performed that included B-mode and Doppler ultrasonography for measurements of arterial diameter, peak-systolic (PS) flow velocities, and end-diastolic (ED) flow velocities along both carotid arteries. All participating subjects provided written informed consents, and the study was performed with the approval of the institutional ethics committee.
We collected demographic and clinical information on all of the study participants, including age, sex, history of ischemic stroke and stroke subtype, and major cardiovascular risk factors. Hypertension was defined as a blood pressure of ≥140/90 mm Hg or treatment with antihypertensive medication, diabetes mellitus was diagnosed by previous use of glucose-lowering medications or a fasting blood glucose of >7.0 mmol/L (126 mg/dL), and hyperlipidemia was defined as previous or current use of lipid-lowering medications or a total cholesterol of ≥6.2 mmol/L (240 mg/dL) or low-density lipoprotein (LDL) cholesterol at ≥4.1 mmol/L (160 mg/dL). Blood samples were drawn in the morning after a 12-hour overnight fast.
Ischemic stroke was defined as a history of cerebral infarction with evidence of an acute focal neurological dysfunction lasting more than 24 h, and cerebral ischemia diagnosed on brain imaging such as computed tomography and/or magnetic resonance imaging. Ischemic strokes are normally categorized into five groups: LAA, cardioembolism, SVO or lacunar infarction (LI), stroke of determined etiology, and stroke of undetermined etiology based upon the diagnostic criteria of the Trial of Org 10172 in Acute Stroke Treatment study.10 The present study only included patients with ischemic stroke of LAA or SVO, thereby excluding other subtypes such as cardioembolism. Recurrent stroke was defined at the time of enrollment by the occurrence of more than one ischemic stroke >21 days after the previous stroke.11 The control group comprised subjects with vascular risk factors but who had no history of vascular events such as myocardial infarction, stroke, or peripheral arterial disease. The subjects in the control group were consulted in an interview with neurologists about headache, small-vessel disease, or silent lacunes while performing regular health screening at Chonbuk National University Hospital. Those who agreed to participate in the study and underwent carotid ultrasonography were enrolled.
Ultrasonography was performed with ECG triggering and using a high-frequency (5-to-12 MHz; 12L5) linear transducer (Terason t3000, Teratech Corporation, Burlington, MA, USA) by a certified neurosonologist (S.K.J.). The ischemic stroke group only included patients who had received carotid duplex ultrasonographic examinations >2 weeks after the acute stroke (interval, 23.1±11.6 days, mean±SD) in order to evaluate patients with stable carotid hemodynamics. Carotid duplex ultrasonography was performed along the CCA and ICA on both the right and left sides. The carotid segments were distinguished in a longitudinal view that was strictly perpendicular to the ultrasound beam, with both vessel walls clearly visualized. The carotid bulb was defined as a segment from the diverging portion of the distal CCA to the beginning portion of the ICA, as recommended previously.12
An atherosclerotic plaque was defined as a focal structure encroaching the arterial lumen by at least 0.5 mm or 50% of the surrounding intima-media thickness (IMT), or as a structure where the thickness measured from the media-adventitia interface to the intima-lumen interface was greater than 1.5 mm.12 Plaque echogenicity was classified into echolucent, echogenic, and calcified, while the plaque surface morphology was classified into smooth or irregular, as reported previously.13 The degree of ICA stenosis in both grayscale and Doppler ultrasonography was stratified into the following six categories according to the PS velocity and the presence of plaque: 1) normal (no stenosis), 2) <50% stenosis, 3) 50-69% stenosis, 4) ≥70% stenosis to near occlusion, 5) near occlusion, and 6) total occlusion, as recommended previously.14
Both the internal diameter and the maximum centerline PS and ED velocities were measured, from which the shear rate was calculated along the carotid arterial segments as described previously.15 Video images were acquired in all carotid segments over six consecutive cardiac cycles. The lumen diameter and IMT were measured at the peak of the R wave. For velocity measurements, a sample volume in the center of the flow was reduced to the smallest possible measurement size (approximately 1 mm), while the Doppler angle was generally maintained at 45±4 degrees, and never exceeded 60 degrees.
Using the PS and ED velocities and diameters along the carotid arterial segments, shear rates (s-1) were determined using the following definition of Newtonian flow:
where V is the maximum centerline (PS or ED) flow velocity, assuming a parabolic velocity distribution across the arterial lumen, and D is the local lumen diameter in the ED phase.15 The blood viscosity (µ) was estimated from a validated model using the hematocrit as follows:16
where H represents the hematocrit [(%)/100]. Finally, the shear stress (dyne/cm2) was determined by multiplying the blood viscosity and shear rate as follows:17
Patients with the following characteristics were excluded: 1) ICA stenosis of >50% on at least one side, 2) carotid artery revascularization performed including carotid endarterectomy and stenting, and 3) ischemic stroke other than LAA and SVO. Patients with ischemic stroke of LAA or SVO and control subjects who had a normal ICA or an ICA with <50% stenosis were included in the present study, since arterial stenoses of <50% do not cause any reduction in flow or pressure distal to the lesion.18
The numbers and percentages of subjects according to the statuses of both ICAs were summarized. Descriptive data for the major clinical characteristics and laboratory findings according to the presence/recurrence of ischemic stroke are expressed as mean±SD or percentage values as appropriate. Analysis of variance was used to assess differences in the continuous variables, and the chi-square test for trend was used to assess the categorical variables. Analysis of covariance was used to evaluate the relationship between adjusted mean values of PS/ED carotid artery shear stress and the three study groups: 1) controls, 2) patients with first-ever ischemic stroke, and 3) patients with recurrent ischemic stroke. Multivariate logistic regression analysis was performed to reveal independent associations between carotid shear stress and ischemic stroke in models adjusted for age, sex, and other potential confounders. Interactions among the three groups and other variables such as sex were assessed. All statistical analyses were conducted using SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA).
ICA shear stress was calculated in 143 (54.0%) control subjects and 122 (46.0%) patients with ischemic stroke of LAA or SVO, who had a normal ICA or an ICA with <50% stenosis. Among the patients with ischemic stroke, 107 (87.7%), and 15 (12.3%) patients were categorized as first-ever and recurrent stroke, respectively, as indicated in Table 1. Male sex, smoking, hypertension, and type 2 diabetes were significantly and positively associated with stroke and its recurrence. In contrast, age, use of statins, LDL cholesterol, and the hematocrit did not differ across the three groups. ICA stenoses of <50% were evenly distributed in the groups. LAA and SVO appeared evenly in patients with first-ever and recurrent ischemic stroke.
Arterial diameters (ED) were significantly larger in the groups with ischemic stroke than the stroke-free group, especially in the right ICA and CCA, as indicated in Table 2. PS and ED blood flow velocities were also significantly lower in patients with ischemic stroke than in the control group. Neither plaque morphology (echogenicity and surface irregularity) nor carotid IMT differed significantly between the groups (data not shown).
Mean values of PS and ED shear stress in both ICAs were significantly lower in patients with ischemic stroke, even after adjusting for age, sex, and major cardiovascular risk factors, as indicated in Table 3 and 4. The PS and ED shear stresses were lowest in patients with recurrent stroke (Table 3) and in those with SVO (Table 4). The shear stress was significantly lower in the left CCA. The PS and ED shear stresses in both ICAs showed independent and negative associations with ischemic stroke after adjusting for the potential confounders, as indicated in Table 5. The interaction terms for carotid shear stress between the three study groups and independent variables including sex, smoking, hypertension, type 2 diabetes, and statin use were not significant (p>0.1).
This study found that the vascular shear stress in carotid arterial segments was significantly lower in patients with ischemic stroke than in the control group. Among the stroke patients, the carotid arterial shear stress was lowest in those with recurrent stroke or SVO. Carotid arterial shear stress was measured in the extracranial portions of the ICA among subjects with normal findings or stenosis of <50%, as assessed ultrasonographically.
Our findings suggest that the milieu of low carotid artery shear stress affects cerebral arteries adversely based on its association with both first-ever and recurrent ischemic strokes. Among the subtypes of ischemic stroke, the carotid artery shear stress was much lower in SVO than LAA, as indicated in Table 4. We previously reported that the blood flow velocities in cerebral arteries were lower in patients with LI (or SVO) than in patients with other ischemic stroke subtypes.19 A low shear stress along the carotid artery was previously reported to be associated with ischemic stroke of LAA, especially on the same side as the affected hemisphere.8 However, that study involved a relatively small number of patients with LAA (n=25), and did not include patients with SVO.
The cross-sectional design of the present study meant that we could not determine whether low carotid artery shear stress was causally related to ischemic stroke. Nevertheless, several mechanisms may contribute to an increased risk of ischemic stroke in regions of low wall shear stress,20 including the production of open gaps due to the endothelial cells becoming more spherical.21 The low shear stress may result in altered physiological responses that include a decrease in endothelial nitric oxide synthase mRNA and protein expression,22 increased release of proinflammatory mediators such as macrophage chemoattractant-1,23 increased uptake of oxidized LDL,24 altered redox state of the vasculature, and altered smooth-muscle-cell gene expression.25 Regions of low arterial shear stress are considered to be vulnerable to future atherosclerotic growth26 and thrombosis evolution.2,3 However, the exact mechanisms underlying how low arterial shear stress affects neurovascular units and cerebrovascular networks need further evaluation.27
Wall shear stress occurs immediately adjacent to the arterial wall. A limitation of the current method includes the use of flow velocities that were obtained in the center of the artery instead of along the vessel wall, and the use of the estimated blood viscosity. Further investigations are required to determine if the arterial shear stress measured with the centerline flow velocity is a parameter for which there are critical threshold values for eliciting vascular diseases or pathologic conditions. Our study involving 122 patients with ischemic stroke showed that the ICA PS shear stress was 16-17 dyne/cm2, which is consistent with a previous report of low carotid arterial shear stresses in 126 subjects with high-risk vascular profiles.20
Therapeutic implications might be considered for improving the carotid and cerebral arterial shear stress when a low arterial shear stress is diagnosed. However, very few experimental trials have investigated the possible beneficial effects of shear-stress-modifying therapy.28 Considering the unstable cerebral and carotid hemodynamics in the acute phase of ischemic stroke, shear-stress-modifying therapy might be more suitable for the subacute or chronic phases of ischemic stroke in order to prevent recurrent attacks. The variability or persistency of subphysiologic arterial shear stress in the transition from the acute to chronic phases requires further evaluation in each subtype of ischemic stroke.
The present study was subject to several limitations. First, the cross-sectional methodology with an interval between the examination of carotid arterial shear stress and ischemic stroke might have hindered the accurate determination of temporal changes in carotid shear stress according to the subtypes of ischemic stroke. Second, the internal diameters of arteries were measured only in the ED phase, so the calculated PS shear stresses might be higher than the true values. Third, the application of the Poiseuille equation in ICA was not consistent with the basic assumption of some patients having mild stenosis (<50%). However, excluding those subjects with mild stenosis did not change the present results markedly (data not shown). The Poiseuille equation can only be applied to a human artery if certain basic assumptions are met. The ICA has no branches along its intracranial course, and if mild stenosis (<50%) affects the local hemodynamics, the present study might have included ICAs with higher shear stresses and thereby have underestimated the true difference. Finally, the blood in the present study was assumed to be Newtonian, even though each carotid shear stress was calculated using patient-specific blood viscosity derived from the hematocrit. Blood actually behaves as a non-Newtonian fluid, in that its viscosity changes with the shear rate.29 Future studies that directly measure the non-Newtonian characteristics of the blood should enhance the accuracy of vascular shear stress measurements and potentially also the clinical utility of carotid arterial shear stress measurements.
ICA shear stresses were significantly lower in patients with ischemic stroke than in the control subjects. Further study is needed to define the causal relationship between carotid arterial shear stress and ischemic stroke.
Figures and Tables
Table 4
Adjusted values of carotid artery shear stress (dyne/cm2) according to subtypes of ischemic stroke

Data are mean±SE values adjusted for age, sex, smoking, hypertension, type 2 diabetes, total cholesterol, LDL cholesterol, hematocrit, and statin medication.
CCA: common carotid artery, ED: end-diastolic, ICA: internal carotid artery, LAA: large-artery atherosclerosis, LDL: low-density lipoprotein, SVO: small-vessel occlusion, PS: peak-systolic.
Acknowledgements
This study was supported by the Research Institute of Clinical Medicine, Chonbuk National University, and the Biomedical Research Institute of Chonbuk National University Hospital.
References
1. Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005; 85:9–23.

2. Giannoglou GD, Soulis JV, Farmakis TM, Farmakis DM, Louridas GE. Haemodynamic factors and the important role of local low static pressure in coronary wall thickening. Int J Cardiol. 2002; 86:27–40.

3. Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009; 15:665–673.

4. Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999; 282:2035–2042.

5. Lee SW, Antiga L, Spence JD, Steinman DA. Geometry of the carotid bifurcation predicts its exposure to disturbed flow. Stroke. 2008; 39:2341–2347.

6. Groen HC, Gijsen FJ, van der Lugt A, Ferguson MS, Hatsukami TS, van der Steen AF, et al. Plaque rupture in the carotid artery is localized at the high shear stress region: a case report. Stroke. 2007; 38:2379–2381.

7. Palm-Meinders IH, Box FM, de Craen AJ, Blauw GJ, van Buchem MA, van der Grond J. Diastolic wall shear stress in the internal carotid artery is associated with different cardiovascular risk factors than systolic wall shear stress. Cerebrovasc Dis. 2009; 28:185–190.

8. Carallo C, Lucca LF, Ciamei M, Tucci S, de Franceschi MS. Wall shear stress is lower in the carotid artery responsible for a unilateral ischemic stroke. Atherosclerosis. 2006; 185:108–113.

9. Rothwell PM, Warlow CP. Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists' Collaborative Group. Stroke. 2000; 31:622–630.

10. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.

11. Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth Community Stroke Study. Stroke. 2004; 35:731–735.

12. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007; 23:75–80.

13. Petersen C, Peçanha PB, Venneri L, Pasanisi E, Pratali L, Picano E. The impact of carotid plaque presence and morphology on mortality outcome in cardiological patients. Cardiovasc Ultrasound. 2006; 4:16.

14. Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis--Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003; 229:340–346.

15. Jiang Y, Kohara K, Hiwada K. Association between risk factors for atherosclerosis and mechanical forces in carotid artery. Stroke. 2000; 31:2319–2324.

16. Guyton AC, Hall JE. Textbook of Medical Physiology. 10th ed. Philadelphia, PA: W.B. Saunders;2000.
17. Cho YI, Kensey KR. Effects of the non-Newtonian viscosity of blood on flows in a diseased arterial vessel. Part 1: Steady flows. Biorheology. 1991; 28:241–262.

18. Schulz UG, Rothwell PM. Major variation in carotid bifurcation anatomy: a possible risk factor for plaque development? Stroke. 2001; 32:2522–2529.

19. Kim JT, Lee SH, Hur N, Jeong SK. Blood flow velocities of cerebral arteries in lacunar infarction and other ischemic strokes. J Neurol Sci. 2011; 308:57–61.

20. Irace C, Cortese C, Fiaschi E, Carallo C, Farinaro E, Gnasso A. Wall shear stress is associated with intima-media thickness and carotid atherosclerosis in subjects at low coronary heart disease risk. Stroke. 2004; 35:464–468.

21. Levesque MJ, Liepsch D, Moravec S, Nerem RM. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis. 1986; 6:220–229.

22. Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, et al. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol. 1997; 17:2479–2488.

23. Sheikh S, Rainger GE, Gale Z, Rahman M, Nash GB. Exposure to fluid shear stress modulates the ability of endothelial cells to recruit neutrophils in response to tumor necrosis factor-alpha: a basis for local variations in vascular sensitivity to inflammation. Blood. 2003; 102:2828–2834.

24. Zhu CH, Ying DJ, Mi JH, Zhu XH, Sun JS, Cui XP. Low shear stress regulates monocyte adhesion to oxidized lipid-induced endothelial cells via an IkappaBalpha dependent pathway. Biorheology. 2004; 41:127–137.
25. Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, et al. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004; 109:520–525.

26. Lee SH, Hur N, Jeong SK. Geometric analysis and blood flow simulation of basilar artery. J Atheroscler Thromb. 2012; 19:397–401.

27. del Zoppo GJ. Virchow's triad: the vascular basis of cerebral injury. Rev Neurol Dis. 2008; 5:Suppl 1. S12–S21.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


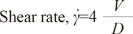
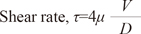




 XML Download
XML Download