Abstract
Patients with dementia and concomitant parkinsonism are frequently encountered in the elderly population. When it comes to young adults, however, coexistence of Alzheimer's disease (AD) and Parkinson's disease (PD) is rare. We described a case of 47-year old man with presenile onset dementia associated with hemiparkinsonism involving the right extremities. Brain biopsy showed neurofibrillary tangles and neuritic plaques, compatible with Alzheimer's disease. Iodine-123 labelled N-(3-iodopropen-2-yl)-2beta-carbomethoxy-3beta-(4-chlorophenyl) tropane ([(123)I]IPT) SPECT, dopamine transporter imaging, revealed a decreased uptake in both basal ganglia, more severe on the left side, particularly the caudal putamen, which is consistent with the finding of idiopathic Parkinson's disease. This case is unique in that damage on the nigrostriatal dopaminergic system in a patient with Alzheimer's disease was demonstrated by a functional neuroimaging study and that early-onset AD and early-onset PD, two rare conditions, coexist in the same individual.
Patients with dementia and concomitant parkinsonism are frequently encountered in the elderly population. There is a long list of differential diagnoses of such a syndrome, often causing a major diagnostic challenge in clinical practice. However, Alzheimer's disease (AD) and Parkinson's disease (PD) rarely coexist in young adults.
We describe clinical manifestations along with the findings of dopamine transporter SPECT and fluorodeoxyglucose (FDG) PET, in a patient with dementia and hemiparkinsonism of presenile onset, who was later diagnosed of AD based on pathologic findings of brain biopsy.
A 47-year-old man was referred to the neurology clinic for cognitive decline and gait disturbance. At the age of 45, he was noted to have easy forgetfulness by his family. His memory got gradually worsened and speech disturbance developed over the same time period. At 46, he had a decreased arm swing and hand dexterity on the right side, which kept him from continuing his job as a police officer and made him retire early. There was no family history of dementia or other neurologic illnesses.
Neurologic examination revealed him to be oriented to person but not to place and time. His facial expression was decreased. Speech was dysarthric and hypophonic. He could not recall any item after 3 minutes of encoding. He could draw simple figures such as circle or rectangle, but could not make it for more complex ones such as copying interlocking pentagons. His Mini-Mental state Examination (K-MMSE) score was 16. The Korean version of Western Aphasia Battery revealed that his speech was nonfluent and effortful with some hesitancy. Naming was markedly impaired with occasional paraphasic errors and neologism. He could only repeat a few words. He had a marked impairment in the comprehension of both spoken and written words, only obeying one step command. He had cogwheel rigidity and hypokinesia in the right upper extremity. There was a 6~8 Hz action tremor in the right hand. No resting tremor was observed. His gait was short-stepped with his right foot dragged and the right arm flexed. A decreased arm swing was noted on the right side. His Unified Parkinson's Disease Rating Scale (UPDRS) scores were as follows: bradykinesia 2/0, rigidity 2/0, resting tremor 0/0, postural tremor 1/0. Hoehn and Yahr staging was 1. He did not show myoclonus, apraxia, or autonomic dysfunction. Neither cognitive fluctuation nor psychiatric symptoms such as visual hallucination and delusion were noticed.
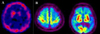
All hematological and biochemical laboratory findings were normal. Apolipoprotein E genotype was E3/3. Brain MRI disclosed diffuse atrophy in both frontotemporoparietal areas, more prominent on the left side. EEG showed intermittent rhythmic delta slowing in both hemispheres. The dopamine transporter image using iodine-123 labelled N-(3-iodopropen-2-yl)-2beta-carbomethoxy-3beta-(4-chlorophenyl) tropane ([(123)I]IPT) SPECT revealed a decreased uptake in both basal ganglia, particularly the caudal putamen, more prominent on the left side (Fig. 1-A). Brain biopsy was performed in left frontal cortex, showing neurofibrillary tangles and neuritic plaques, which is compatible with Alzheimer's disease (Fig. 2). No Lewy bodies were found.
After treatment with donepezil and L-dopa, bradykinesia and gait difficulty improved, whereas his dementia relentlessly progressed. Brain FDG PET scan, 3 years after the onset of symptoms, showed markedly decreased glucose metabolism in both frontal, parietal, and temporal lobes with preserved metabolic activity in the primary motor and sensory cortices including primary visual cortex, consistent with advanced Alzheimer's disease (Fig. 1-B). Four years after onset of dementia, he became bedridden and died of pneumonia subsequently.
Extrapyramidal symptoms (EPSs) are often presented in patients with AD.1-3 The relationship between EPSs and AD is complex and differs depending on the disease or the clinical setting. Our patient's parkinsonism developed about 1 year after cognitive impairment and was restricted to the right side. In addition, the findings of [(123)I]IPT SPECT, dopamine transporter imaging, showed a profile of a decreased uptake in the caudal putamen, which is typically found in patients with idiopathic Parkinson's disease (IPD). The abnormality of dopamine transporter sites was asymmetrical and more severe in the left putamen than in the right one, compatible with the clinical finding of right hemiparkinsonism. The possibility of dementia with Lewy bodies (DLB) could be ruled out due to the absence of fluctuating cognition and psychiatric manifestations on a history and the lack of cortical Lewy bodies on brain biopsy. Other differential diagnoses of dementia with parkinsonism such as corticobasal degeneration, progressive bulbar palsy, multiple system atrophy, normal pressure hydrocephalus, or Binswanger's disease were easily excluded based on clinical features and neuroimaging changes.
The investigation on damage to the dopamine system in IPD, AD, and AD with EPSs revealed different patterns among them.4 In AD, the loss of dopamine transporter sites was restricted to nucleus accumbens. In AD with EPSs, it was most prominent in the rostral caudate and putamen with the caudal striatum least affected. On the other hand, in IPD, the caudal striatum was the most pronounced area of dopamine transporter site loss.
We postulated that our patient has AD and concomitant IPD rather than AD with accompanying EPSs for the following reasons. First, parkinsonism developed after dementia in a short interval when EPSs are not usually expected in AD. Secondly, parkinsonism of our patient was asymmetric similar to IPD. Thirdly, the dopamine transporter image showed a decreased uptake in the caudal putamen, which is exactly the same as in IPD.4,5 Finally, our patient responded to antiparkinsonian medications. We cannot completely exclude the possibility that the EPSs in our patient might be simply the manifestation of AD, because the autopsy was not performed and the question was unresolved as to whether the substantia nigra has NFTs or LBs.6 So, the precise pathogenic mechanism of EPSs of our patient remained unanswered. There have been many reports of cases in which the pathologic features of both AD and PD are encountered in the same individual.7 Autopsy findings revealed that nigral pathology consistent with IPD was identified in 80% of patients with AD with EPSs, suggesting the common pathogenetic mechanism involved in AD and PD.7
This case is unique in that damage on the nigrostriatal dopaminergic system in a patient with Alzheimer's disease was demonstrated by a functional neuroimaging study and that early-onset AD and PD, two rare conditions, coexist in the same individual.
Figures and Tables
References
1. Morris JC, Drazner M, Fulling K, Grant EA, Goldring J. Clinical and pathological aspects of parkinsonism in Alzheimer's disease. Arch Neurol. 1989. 46:651–657.

2. Stern Y, Albert M, Brandt J, Jacobs DM, Tang MX, Marder K, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer's disease: prospective analyses from the predictors study. Neurology. 1994. 44:2300–2307.

3. Lopez OL, Wisnieski SR, Becker JT, Boller F, DeKosky ST. Extrapyramidal signs in patients with probable Alzheimer disease. Arch Neurol. 1997. 54:969–975.

4. Murray AM, Weihmueller FB, Marshall JF, Hurting HI, Gottleib GL, Joyce JN. Damage to systems differs between parkinson's disease and Alzheimer's disease with parkinsonism. Ann Neurol. 1995. 37:300–312.

5. Piggott MA, Marshall EF, Thomas N, Lloyd S, Court JA, Jaros E, et al. Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer's and parkinson's disease: rostrocaudal distribution. Brain. 1999. 122:1449–1468.

6. Liu Y, Stern Y, Chun MR, Jacobs DM, Yau P, Goldman JE. Pathological correlates of extrapyramidal signs in Alzheimer's disease. Ann Neurol. 1997. 41:368–374.

7. Perl DP, Olanow CW, Calne D. Alzheimer's disease and Parkinson's disease: distinct entities or extremes of spectrum of neurodegeneration? Ann Neurol. 1998. 44:Suppl 1. S19–S31.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download