1. Perkin GD. Cerebral venous thrombosis: developments in imaging and treatment. J Neurol Neurosurg Psychiatry. 1995. 59:1–3.

2. Bousser M-G, Barnett HJM. Mohr JP, Choi DW, Grotta JC, Weir B, Wolf AP, editors. Cerebral venous thrombosis. Stroke: Pathophysiology, Diagnosis, and Management. 2004. 3rd ed. Philadelphia: Churchill Livingstone;301–325.

3. Einhaupl KM, Villringer A, Meister W, Mehraein S, Garner C, Pellkofer M, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991. 338:597–600.

4. Stam J, de Bruijn S, deVeber G. Anticoagulation for cerebral sinus thrombosis. Stroke. 2003. 34:1054–1055.

5. DiNubile MJ. Septic thrombosis of the cavernous sinuses. Arch Neurol. 1988. 45:567–572.

6. Feldenzer JA, Bueche MJ, Venes JL, Gebarski SS. Superior sagittal sinus thrombosis with infarction in sickle cell trait. Stroke. 1987. 18:656–660.

7. Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997. 337:688–698.

8. Bergqvist D, Burmark US, Flordal PA, Frisell J, Hallbook T, Hedberg M, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995. 82:496–501.

9. Torholm C, Broeng L, Jorgensen PS, Bjerregaard P, Josephsen L, Jorgensen PK, et al. Thromboprophylaxis by low-molecular-weight heparin in elective hip surgery. A placebo controlled study. J Bone Joint Surg Br. 1991. 73:434–438.

10. Lensing AW, Prins MH, Davidson BL, Hirsh J. Treatment of deep venous thrombosis with low molecular-weight heparins. A meta-analysis. Arch Intern Med. 1995. 155:601–607.

11. Kher A, Samama MM. Primary and secondary prophylaxis of venous thromboembolism with low molecular-weight heparins: prolonged thromboprophylaxis, an alternative to vitamin K antagonists. J Thromb Haemost. 2005. 3:473–481.

12. SYNERGY Executive Committee. Superior Yield of the New strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa inhibitors. The SYNERGY trial: study design and rationale. Am Heart J. 2002. 143:952–960.
13. Blazing MA, de Lemos JA, White HD, Fox KA, Verheugt FW, Ardissino D, et al. Safety and efficacy of enoxaparin vs unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes who receive tirofiban and aspirin: a randomized controlled trial. JAMA. 2004. 292:55–64.

14. Biller J, Love BB. Bradely WG, Daroff RB, Fenichel GH, Jankovic J, editors. Vascular disease of the venous system. Neurology in Clinical Practice. 2005. 4th ed. Philadelphia: Elsevier Inc.;1243–1245.
15. Brucker AB, Vollert-Rogenhofer H, Wagner M, Stieglbauer K, Felber S, Trenkler J, et al. Heparin treatment in acute cerebral sinus venous thrombosis: a retrospective clinical and MR analysis of 42 cases. Cerebrovasc Dis. 1998. 8:331–337.

16. Ameri A, Bousser MG. Cerebral venous thrombosis. Neurol Clin. 1992. 10:87–111.

17. Quinlan DJ, McQuillan A, Eikelboom JW. Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2004. 140:175–183.

18. Siragusa S, Cosmi B, Piovella F, Hirsh J, Ginsberg JS. Low-molecular-weight heparins and unfractionated heparin in the treatment of patients with acute venous thromboembolism: results of a meta-analysis. Am J Med. 1996. 100:269–277.

19. Leizorovicz A, Mismetti P. Preventing venous thromboembolism in medical patients. Circulation. 2004. 110:IV13–IV19.

20. de Bruijn SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999. 30:484–488.

21. Stam J, Lensing AW, Vermeulen M, Tijssen JG. Heparin treatment for cerebral venous and sinus thrombosis. Lancet. 1991. 338:1154.

22. Enevoldson TP, Russell RWR. Heparin treatment in sinus venous thrombosis. Lancet. 1991. 338:1153–1154.

23. Das P, Moliterno DJ. Fractionating heparins and their clinical trial data--something for everyone. JAMA. 2004. 292:101–103.

24. Hirsh J. Heparin. N Engl J Med. 1991. 324:1565–1574.

25. van den Belt AG, Bossuyt PM, Prins MH, Gallus AS, Buller HR. Replacing inpatient care by outpatient care in the treatment of deep venous thrombosis--an economic evaluation. TASMAN Study Group. Thromb Haemost. 1998. 79:259–263.

26. Rodger M, Bredeson C, Wells PS, Beck J, Kearns B, Huebsch LB. Cost-effectiveness of low-molecular-weight heparin and unfractionated heparin in treatment of deep vein thrombosis. CMAJ. 1998. 159:931–938.
27. Iorio A, Guercini F, Pini M. Low-molecular-weight heparin for the long-term treatment of symptomatic venous thromboembolism: meta-analysis of the randomized comparisons with oral anticoagulants. J Thromb Haemost. 2003. 1:1906–1913.

28. Ockelford PA, Patterson J, Johns AS. A double-blind randomized placebo controlled trial of thromboprophylaxis in major elective general surgery using once daily injections of a low molecular weight heparin fragment (Fragmin). Thromb Haemost. 1989. 62:1046–1049.

29. Lindmarker P, Holmstrom M, Granqvist S, Johnsson H, Lockner D. Comparison of once-daily subcutaneous Fragmin with continuous intravenous unfractionated heparin in the treatment of deep vein thrombosis. Thromb Haemost. 1994. 72:186–190.

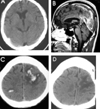
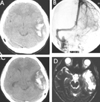






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download