Abstract
Graft-versus-host disease (GVHD) is a common complication of bone marrow transplantation (BMT) that can be classified as acute or chronic. Chronic GVHD, which usually occurs more than 3 months after BMT, includes typical lichenoid or sclerodermatous lesions. Psoriasiform eruption is a rare clinical manifestation of chronic GVHD, and there have been no reports of psoriasiform chronic GVHD associated with hemophagocytic lymphohistiocytosis. A 33-year-old woman who was diagnosed with hemophagocytic lymphohistiocytosis 10 years ago visited our outpatient clinic with psoriasiform eruption over her entire body. She underwent allogeneic BMT 7 months previously from her sibling. Skin biopsy was performed on the lesion, and the histological features suggested GVHD. The psoriasiform lesions improved with narrow-band ultraviolet B phototherapy, with secondary vitiligo remaining on the corresponding locations.
Graft-versus-host disease (GVHD) is a frequent complication of bone marrow transplantation (BMT), which can be classified as acute or chronic. The skin is the most frequently affected organ in GVHD. The characteristic skin manifestations of acute GVHD occur within 3 months after BMT and include maculopapular rash, whereas those of chronic GVHD occur more than 3 months after BMT and include lichenoid or sclerodermatous lesions. Although the disease may show various cutaneous manifestations, the association between psoriasiform eruptions and GVHD has rarely been described except in limited clinical situations123.
Herein, we report a rare case of generalized psoriasiform eruption associated with chronic GVHD that resulted in secondary vitiligo in a patient with hemophagocytic lymphohistiocytosis (HLH).
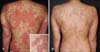
The patient was a 33-year-old woman with a 10-year history of HLH without any history of skin diseases and/or familial history of psoriasis and HLH. Because she had HLH involvement in the bone marrow and liver, she received an allogeneic BMT in January 2014 from a human leukocyte antigen-matched male sibling with no documented history of psoriasis. The patient received busulfan (3.2 mg/kg daily on days −7 and −6), fludarabine (30 mg/m2 daily on days −7 to −2), and antithymocyte globulin (1.5 mg/kg daily on days −3 to −1) as a conditioning regimen and oral tacrolimus (0.2 mg/kg daily) for GVHD prophylaxis starting 1 day before BMT. On day 119 after the BMT, skin lesions and mild pruritus developed on her abdomen. Her condition was diagnosed as a lichenoid eruption at another clinic, but antihistamine and topical methylprednisolone were ineffective. Her status worsened and she visited our clinic on day 187. After 2 weeks of oral prednisolone (0.3 mg/kg daily) with topical desoxymethasone, the skin lesions improved, and we started tapering the medication. However, the eruptions abruptly recurred in the form of erythematous lesions across her whole body including the scalp, palms, and soles except the nails, without other general symptoms on day 201 just after the dosage was decreased. The eruptions were composed of papulosquamous lesions with whitish scales, and the clinical features were similar to those of psoriasis (Fig. 1A).
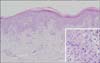
A laboratory examination including liver function testing found that all markers were within normal ranges. A histopathological examination of the arm indicated interface dermatitis with perivascular lymphocytic infiltration in the upper dermis, keratinocyte apoptosis, exocytosis of lymphocytes, hyperkeratosis, and parakeratosis (Fig. 2). However, no typical histologic findings of psoriasis such as spongiform pustules of Kogoj, Munro's microabscesses, hypogranulosis, or rete ridge elongation were observed.
Overall, we found discrepancies between the clinical and histological findings. Although the clinical findings suggested psoriasiform lesions, the histological findings suggested GVHD, for example, dyskeratotic cells in the epidermis. Based on these findings, a diagnosis of mild chronic GVHD with a psoriasiform skin manifestation was made. Skin was the only involved organ in this case. Oral prednisolone (0.5 mg/kg daily) and narrow-band ultraviolet B phototherapy 3 times per week were started. These treatments were effective and the skin lesions were significantly improved, with widespread hypopigmented patches remaining (Fig. 1B).
The characteristic cutaneous manifestations of acute GVHD, which generally occur within 3 months after BMT, include maculopapular exanthema and perifollicular papular lesions. In contrast, chronic GVHD, which usually occurs more than 3 months after BMT, includes typical lichenoid or sclerodermatous lesions with poikilodermal elements.
Although cutaneous involvement is common in GVHD, psoriasiform eruptions after BMT are rare. Several cases showing the development of psoriasis after BMT from donors with a history of psoriasis or resolution of psoriasis after BMT for chronic myelogenous leukemia from a normal donor have been reported45, suggesting an adoptive transfer of susceptibility or disease-inducible immunity to psoriasis from a donor with a genetic background of psoriasis.
Three other reports of psoriasiform eruptions associated with GVHD have been reported in the literature678. The unusual psoriasiform skin presentation as described here could be a diagnostic challenge to clinicians. Even in HLH, the development of chronic GVHD is relatively rare (19%) compared to that of other conditions (30% from matched and 70% from mismatched donors)9. There are no other previous reports of psoriasiform chronic GVHD in a patient with HLH after BMT. Taking a detailed history was essential in considering GVHD, which was indicated by histopathological findings.
GVHD and psoriasis share common immunological features. Both diseases are T-cell–mediated diseases displaying a T helper cell type 1 cytokine secretion profile, and both exhibit elevated human leukocyte antigen (HLA)-DR antigen expression in the lesional epidermal keratinocytes6. T-cell–directed immunosuppressants effectively control the disease activity of psoriasis, and psoriasis skin lesions are fully induced when T cells from patients with psoriasis are injected into symptomless psoriasis skin engrafted onto severe combined immunodeficiency mice10. Although it is unclear why this patient presented with psoriasiform eruption as a skin lesion of chronic GVHD, one possible explanation is that the interaction between donor lymphocytes and recipient keratinocytes during graft-versus-host reactions induced keratinocyte hyperproliferation.
In addition, Langerhans cell (LC) counts have been reported to be decreased in the lesional skin of patients with GVHD and psoriasis6. Although the association of immunological abnormalities relevant to local antigen presentation and psoriasiform eruption remains to be fully understood, it might be explained by the evidence of exacerbation of psoriasis in patients with acquired immunodeficiency syndrome accompanied by a reduced number of epidermal LCs11 or the enhancement of inflammatory skin reactions when epidermal LCs are depleted in the epidermis12. Thus, the decrease in LC counts also might play a role in the formation of psoriasiform eruption.
Secondary vitiligo can be explained by autoimmune reactions triggered by chronic GVHD, and the rather rapid repigmentation is probably due to the cessation of this process after the active inflammation is resolved. The association of GVHD with various autoimmune conditions strengthens the probability of vitiligo as being a part of the autoimmune process on the basis of chronic GVHD13. GVHD appears to participate in the destruction of melanocytes by skin-homing autoreactive melanocyte-specific cytotoxic T lymphocytes. Furthermore, the influence of sex mismatch on the development of vitiligo, male donor to female recipient in our case, is consistent with previous reports1415.
Herein, we present a case of a novel skin manifestation of chronic GVHD. We would like to call this cutaneous feature "psoriasiform chronic cutaneous GVHD". Further accumulation of similar cases will be needed to enrich the understanding of the variety of immunological events and to clarify the mechanism associated with GVHD. We also emphasize the importance of maintaining a high index of suspicion of GVHD, even for uncommon lesions on the skin of patients with a history of BMT.
Figures and Tables
Fig. 1
(A) Generalized erythematous papulosquamous lesions with whitish scales on the trunk (inlet) and erythematous papular lesion with whitish scales. (B) Improved skin lesions with remaining widespread hypopigmentation 8 weeks after the start of therapy. Some hyperpigmented spots corresponding to hair follicles were suspected to be due to repigmentation.
References
1. Snowden JA, Heaton DC. Development of psoriasis after syngeneic bone marrow transplant from psoriatic donor: further evidence for adoptive autoimmunity. Br J Dermatol. 1997; 137:130–132.

2. Adkins DR, Abidi MH, Brown RA, Khoury H, Goodnough LT, Vij R, et al. Resolution of psoriasis after allogeneic bone marrow transplantation for chronic myelogenous leukemia: late complications of therapy. Bone Marrow Transplant. 2000; 26:1239–1241.

3. Ahn HS, Park HJ, Lee JY, Cho BK. A case of chronic cutaneous graft versus host disease with the clinical features of exfoliative dermatitis. Ann Dermatol. 2009; 21:319–322.

4. Masszi T, Farkas A, Remenyi P, Lueff S, Batai A, Krivan G, et al. Ten-year remission of psoriasis after allogeneic but not autologous bone marrow transplantation. Dermatology. 2006; 212:88–89.

5. Slavin S, Nagler A, Varadi G, Or R. Graft vs autoimmunity following allogeneic non-myeloablative blood stem cell transplantation in a patient with chronic myelogenous leukemia and severe systemic psoriasis and psoriatic polyarthritis. Exp Hematol. 2000; 28:853–857.

6. Kawakami Y, Oyama N, Nakamura K, Kaneko F, Kikuta A, Suzuki H. Psoriasiform eruption associated with graft-versus-host disease. Acta Derm Venereol. 2007; 87:436–438.

7. Matsushita T, Hasegawa M, Shirasaki F, Fujimoto M, Yamazaki H, Sato S, et al. A case of acute cutaneous graft-versus-host disease mimicking psoriasis vulgaris. Dermatology. 2008; 216:64–67.

8. Taguchi S, Kawachi Y, Fujisawa Y, Nakamura Y, Furuta J, Otsuka F. Psoriasiform eruption associated with graft-versus-host disease. Cutis. 2013; 92:151–153.
9. Jordan MB, Filipovich AH. Hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis: a journey of a thousand miles begins with a single (big) step. Bone Marrow Transplant. 2008; 42:433–437.

10. Nickoloff BJ, Wrone-Smith T. Injection of pre-psoriatic skin with CD4+ T cells induces psoriasis. Am J Pathol. 1999; 155:145–158.

11. Zemelman V, Van Neer F, Roberts N, Patel P, Langtry J, Staughton RC. Epidermal Langerhans cells, HIV-1 infection and psoriasis. Br J Dermatol. 1994; 130:307–311.

12. Grabbe S, Steinbrink K, Steinert M, Luger TA, Schwarz T. Removal of the majority of epidermal Langerhans cells by topical or systemic steroid application enhances the effector phase of murine contact hypersensitivity. J Immunol. 1995; 155:4207–4217.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download