Dear Editor:
Basal cell carcinoma (BCC) is the most common form of skin cancer in the world1. Superficial BCC (sBCC) is characterized by basaloid tumor cells confined to the epidermis or superficial dermis and there is a horizontal spread rather than deeper dermal involvement2. Although it can be locally invasive and destructive, it is typically slow growing and rarely metastatic1. For such characteristics of sBCC, although surgical excision is the definitive treatment, topical therapies such as 5-fluorouracil, 5% imiquimod cream or photodynamic therapy can be alternatives for sBCC, especially in patients who are poor candidates for surgery. We herein report a case of sBCC successfully treated with two cycles of topical ingenol mebutate (IM) gel 0.015%.
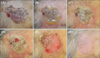
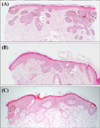
An 84-year-old Korean woman presented with a pruritic, solitary, 5.2×4.5-cm-sized, irregular-shaped, blackish patch on the forehead for 5 years (Fig. 1A). Histologic examination was consistent with sBCC (Fig. 2A). Considering the old age of patient and preference for non-surgical treatment, we attempted topical application of IM gel 0.015% once daily for 4 days consecutively on the lesion within 5 mm margin. Considering sBCC being more locally invasive than actinic keratosis (AK), we applied the gel for one day longer than the conventional 3-day regimen for AK. On the second day, she experienced severe pain and flaking/blistering/erythema extending beyond the application site (Fig. 1B). From the third day, the pain was gradually relieved and erosive patch developed after blister rupture (Fig. 1C~E). The local reaction lasted almost 2 weeks and then we observed almost complete resolution after 2 months from the treatment. Ten weeks after the treatment (Fig. 1F), we performed a follow up biopsy and the result showed clearance of the tumor by moderate depth but remaining lesion was observed (Fig. 2B). A second application of IM gel 0.015% was performed. Interestingly, the local skin reaction seemed to occur less intensely than the previous cycle. Ten weeks after the second treatment, the entire lesion was clinically resolved and follow up biopsies showed complete histological clearance (Fig. 2C). At additional follow up after 3 months, there was no evidence of recurrence.
IM is a novel agent, extracted from the sap of the plant Euphorbia peplus, which is known to have therapeutic effects on various cutaneous neoplasms including warts, corns and cancerous lesions3. Clinical studies have proven its efficacy and safety, leading to the U.S. Food and Drug Administration approval for the therapy of AK34. Topical IM gel is well tolerated in the treatment of AK, with mild to moderate severity of adverse events including erythema, scaling, crusting, swelling, vesiculation/pustulation and erosion/ulceration. IM has a dual mode of action, facilitating cell necrosis, and producing antibodies against the tumor, resulting in the destruction of dysplastic cells3. This is the first histologically proven case of sBCC successfully treated with two cycles of IM 0.015% reported in the published work. We suggest that IM 0.015% could be a safe and effective treatment option for sBCC, with a short treatment course, successful efficacy and cosmetic outcomes.
Figures and Tables
References
1. Raasch BA, Buettner PG, Garbe C. Basal cell carcinoma: histological classification and body-site distribution. Br J Dermatol. 2006; 155:401–407.

2. Chen CC, Chen CL. Clinical and histopathologic findings of superficial basal cell carcinoma: a comparison with other basal cell carcinoma subtypes. J Chin Med Assoc. 2006; 69:364–371.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download