Abstract
Eccrine syringofibroadenoma (ESFA) is a rare benign cutaneous adnexal lesion characterized by a hyperplastic epithelium and eccrine ductal differentiation. In the present case, a 73-year-old Korean male presented with symmetrical numerous widespread, pinkish nodules with a cobblestone appearance over both legs for 2 years. He had a history of generalized erythematous scaly patches over his entire body for 20 years. On histopathologic findings, diagnosis of ESFA was confirmed. Our unusual and interesting case emphasizes the first report described one case in which multiple cobblestone like ESFAs arising from long-standing exfoliative dermatitis.
Eccrine syringofibroadenoma (ESFA) is an uncommon benign cutaneous adnexal lesion characterized by a hyperplastic epithelium and eccrine ductal differentiation that predominantly occurs in patients older than 40 years. It has a strikingly polymorphous clinical presentation ranging from a solitary papule or nodule to multiple lesions with a linear arrangement; the latter is often referred to as eccrine syringofibroadenomatosis1. Although ESFA has a variable clinical presentation, the histological findings are unique2. It is characterized as a distinct tumor composed of proliferating, anastomosing cords of monomorphous epithelial cells harboring eccrine ductal formations admixed within an inflammatory fibrovascular stroma. Due to clinical polymorphism, it is still unclear whether ESFA is a neoplasm, hamartoma, or reactive eccrine hyperplasia3. We describe an unusual and interesting case in which multiple cobblestone like ESFAs occurred in the setting of chronic exfoliative dermatitis.
A 73-year-old Korean male presented to our dermatologic clinic with generalized erythematous scaly patches over his entire body for approximately 20 years. His medical history was significant for diabetes mellitus and hypertension, and there was no family history of similar skin lesions. Physical examination revealed symmetrical numerous widespread, pinkish nodules with a cobblestone appearance over both legs for 2 years (Fig. 1). There was no trauma history over the lower extremities. Laboratory findings, including blood urea nitrogen, creatinine, urine protein, fasting glucose, and total cholesterol level were mildly elevated due to his diabetes mellitus. Punch biopsies from two different lesional sites (patch and nodule) were performed. Histopathological examination of the patchy lesion revealed acanthosis and hyperkeratosis with exfoliation and perivascular inflammatory infiltration in the superficial dermis (Fig. 2A), consistent with exfoliative dermatitis. A histopathological examination of the nodular lesion showed reticular, thin anastomosing strands of uniform cuboidal epithelial cells growing into the dermis with epithelial cords embedded in a fibrovascular stroma (Fig. 2B). Given this information, the diagnosis of ESFA was made. In a causal relationship, we suggest ESFA resulted from epidermal remodeling of exfoliative dermatitis because lesions were distributed broadly and symmetrically, and the erythroderma observed over the lower extremities was more severe than that at other sites, resulting in transmutable damage in the epidermis.
Starink4 suggested that ESFA should be classified into four main subtypes: (1) solitary ESFA, (2) multiple ESFA with hidrotic ectodermal dysplasia (Schopf syndrome), (3) multiple ESFA without associated cutaneous abnormalities, also called eccrine syringofibroadenomatosis, and (4) non-familial unilateral linear ESFA, sometimes referred to as nevoid ESFA. In 1997, French5 suggested reactive ESFA as the fifth subtype of ESFA that appears to result from eccrine ductal remodeling associated with numerous entities. It has been reported in association with chronic skin ulcers, burn scars, lepromatous neuropathy, venous stasis, bullous pemphigoid, erosive palmoplantar lichen planus, peristomal dermopathy, or nevus sebaceous678. Here we suggest first report described one case arising from long-standing exfoliative dermatitis.
The pathologies are characterized by repetitive damage and regrowth of skin structures within affected sites and suggest that ESFA in the vicinity occur as a consequence of recurrent eccrine ductal lesions. Indeed, eccrine ductal proliferation as a consequence of prior ductal disruption is a common response observed during wound healing and in inflammatory or neoplastic skin disorders9. In addition, the increased mast cells observed in several of these cases1011 are a characteristic feature of healing wounds and also suggest that ESFA may be a response of the eccrine duct to ongoing tissue remodeling. The histological presentation of ESFA is unique, and the ultrastructural and immunohistochemical features reported in the literature support acrosyringeal11 or intradermal eccrine duct differentiation1213. However, because of the clinical polymorphism, it is still unclear whether ESFA is a neoplasm, hamartoma, or reactive eccrine hyperplasia.
In immunohistochemical studies, cytokeratin expression in ESFA has stressed the pathogenic role of dysregulated differentiation12. ESFA epithelium appears to have undergone differentiation toward the eccrine excretory portion, with broad bands and thin strands exhibiting a similar staining pattern to the acrosyringium-dermal duct junction and dermal duct proper, respectively12. Saggini and Mully14 suggested that Pleckstrin homology-like domain, family A, member 1 protein (PHLDA-1), an anti-apoptotic protein expressed by hair follicle bulge keratinocytes, is expressed by the basal layer of ESFA strands and cords. Increased level of PHLDA-1 has been demonstrated in the hyperplastic epithelial networks of fibroepithelioma of Pinkus (which resembles that of ESFA) and the basal layer of the junction between the acrosyringium and eccrine dermal duct15.
In some reports, we observed malignant transformation of ESFA161718. In reviewing the reported cases, the following unifying features were identified: (1) patients age (averagely seventh or eighth decade), (2) male patients, (3) involving the extremities, and (4) concerned clinical manifestation including new growth, ulceration and crusting, and persistent disease despite extensive treatment. Notably, there are no reports of malignancy occurring in the setting of reactive ESFA developing from inflammatory dermatoses.
The treatment of ESFA relies on the number, area, location and resectability of the lesions. Solitary ESFA could be completely cured by surgical excision19. Additionally, complete excision seems to be the appropriate treatment for ESFA associated with malignant risk in the limited reports available161718. If skin lesions are difficult to be excised, multiple biopsy is necessary to exclude malignant transformation. Spontaneous regression of reactive ESFA after successful treatment of the underlying inflammatory condition has been described, corroborating the notion that reactive ESFA be regarded as an example of secondary, non-autonomous epidermal hyperplasia15. Recently, one case obtained good results for treatment using pusing pulsed dye in incurable ESFA20.
In this case we reported an interesting clinical manifestation of reactive ESFA, namely widespread cobblestone-like nodules on both legs. It seems that this unique feature would be given repetitive damage-repair cycles causing dysregulation of epidermal growth and differentiation due to previous exfoliative dermatitis. If complete excision is not possible because of its size, number or anatomic problem, just like in this case, histopathologic examination and close clinical follow-up is recommended.
Figures and Tables
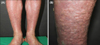 | Fig. 1(A, B) Clinical images of patient. Symmetrical numerous widespread, pinkish nodules with a cobblestone appearance over both legs. |
 | Fig. 2Histopathologically, acanthosis, hyperkeratosis with exfoliation and perivascular inflammatory infiltration are observed in the superficial dermis (H&E, ×100). (B) Reticular, thin anastomosing strands of uniform cuboidal epithelial cells indicated in the arrow are growing into the dermis with epithelial cords embedded in a fibrovascular stroma (H&E, ×200). |
References
1. Lui H, Stewart WD, English JC, Wood WS. Eccrine syringofibroadenomatosis: a clinical and histologic study and review of the literature. J Am Acad Dermatol. 1992; 26:805–813.

2. Cota C, Ferrara G, Amantea A, Donati P. Eccrine syringofibroadenoma and clear cell acanthoma: an association by chance? Am J Dermatopathol. 2011; 33:195–198.

3. Xu XL, Zhang W, Liu WD, Sun JF. A case of multiple eccrine syringofibroadenoma mimicking verruca vulgaris. J Dermatol. 2013; 40:665–666.

4. Starink TM. Eccrine syringofibroadenoma: multiple lesions representing a new cutaneous marker of the Schöpf syndrome, and solitary nonhereditary tumors. J Am Acad Dermatol. 1997; 36:569–576.

5. French LE. Reactive eccrine syringofibroadenoma: an emerging subtype. Dermatology. 1997; 195:309–310.

6. Utani A, Yabunami H, Kakuta T, Endo H, Shinkai H. Reactive eccrine syringofibroadenoma: an association with chronic foot ulcer in a patient with diabetes mellitus. J Am Acad Dermatol. 1999; 41:650–651.

7. Ichikawa E, Fujisawa Y, Tateishi Y, Imakado S, Otsuka F. Eccrine syringofibroadenoma in a patient with a burn scar ulcer. Br J Dermatol. 2000; 143:591–594.

8. Cho E, Lee JD, Cho SH. A case of reactive eccrine syringofibroadenoma. Ann Dermatol. 2011; 23:70–72.

9. Mehregan AH. Proliferation of sweat ducts in certain diseases of the skin. Am J Dermatopathol. 1981; 3:27–31.

10. Gambini C, Rongioletti F, Semino MT, Rebora A. Solitary eccrine syringofibroadenoma (or eccrine syringofibroadenomatous hyperplasia?) and diabetic polyneuropathy. Dermatology. 1996; 193:68–69.

11. Sueki H, Miller SJ, Dzubow LM, Murphy GF. Eccrine syringofibroadenoma (Mascaro): an ultrastructural study. J Cutan Pathol. 1992; 19:232–239.

12. Ohnishi T, Suzuki T, Watanabe S. Eccrine syringofibroadenoma. Report of a case and immunohistochemical study of keratin expression. Br J Dermatol. 1995; 133:449–454.

13. Takeda H, Mitsuhashi Y, Yoshikawa K, Katagata Y, Kondo S. Eccrine syringofibroadenoma: report of a case and analysis of cytokeratin expression. Dermatology. 1998; 196:242–245.

14. Saggini A, Mully T. Reactive eccrine syringofibroadenomatosis secondary to primary cutaneous amyloidosis: a novel association. J Cutan Pathol. 2014; 41:380–385.

15. Tey HL. Characterizing the nature of eccrine syringofibroadenoma: illustration with a case showing spontaneous involution. Clin Exp Dermatol. 2009; 34:e66–e68.

16. Bjarke T, Ternesten-Bratel A, Hedblad M, Rausing A. Carcinoma and eccrine syringofibroadenoma: a report of five cases. J Cutan Pathol. 2003; 30:382–392.

17. González-Serva A, Pró-Rísquez MA, Oliver M, Caruso MG. Syringofibrocarcinoma versus squamous cell carcinoma involving syringofibroadenoma: is there a malignant counterpart of Mascaro's syringofibroadenoma? Am J Dermatopathol. 1997; 19:58–65.

18. Lele SM, Gloster ES, Heilman ER, Chen PC, Chen CK, Anzil AP, et al. Eccrine syringofibroadenoma surrounding a squamous cell carcinoma: a case report. J Cutan Pathol. 1997; 24:193–196.

19. Mcniff J. Benign tumors with apocrine and eccrine differentiation. In : LeBoit PE, Weedon D, Sarason A, editors. World Health Organization Classification of Tumors. Pathology and genetics of skin tumors. IARC Press: Lyon;2006. p. 142.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download