This article has been corrected. See "Erratum: Late-Onset Complication of Fillers: Paraffinoma of the Lower Eyelids Clinically Mimicking Xanthelasma" in Volume 29 on page 135.
Abstract
Injectable poly-L-lactic acid (PLLA) is world-famous filler used in lipoatrophy and facial rejuvenation because of its collagen neogenesis effect which leads to gradual volume restoration. Until recently, quite a number of unwanted adverse events of PLLA have been reported. However, to the best of our knowledge, paraffinoma as a complication of PLLA has never been reported. We herein describe the first case of paraffinoma after Sculptra® injection and propose its possible mechanism.
Injectable poly-L-lactic acid (PLLA, Sculptra; Dermik Laboratories, Paris, France) is a synthetic gel polymer composed of PLLA and suspensions1234. It is a world-famous filler being currently used in lipoatrophy and facial rejuvenation because of its biocompatibility, resorbable nature, and easy availability1234. Injectable PLLA has been used for more than a decade in Europe and was approved in the United States in 200414. A number of side effects of PLLA have been reported, and hence, long-term safety and reversibility of its side effects have been questioned245. Known late-onset complications of PLLA in the literature include nodule, granuloma, pseudoabscess, angioedema, and skin induration245; however, to the best of our knowledge, paraffinoma as a complication of PLLA has never been reported. We herein report the case of a patient who presented with paraffinoma that clinically mimicked xanthelasma in both lower eyelids 2 years after Sculptra® injection.
A 47-year-old woman presented with an 8-month history of yellowish plaques in both lower eyelids (Fig. 1). She reported undergoing filler injection in both lower eyelids 2 years previously at an aesthetic clinic, and she clearly remembered Sculptra® being used as the filler. She denied being injected with anything else or undergoing any other aesthetic procedure in the lesional area. The lesions were asymptomatic, and she denied any adverse events other than the plaque lesions after the procedure.
Her medical history included allergic rhinitis for which she took oral steroid intermittently. She denied history of other diseases or medications. She had no family history of xanthelasma, hyperlipidemia, or other related metabolic disorders.
Initially, we clinically suspected xanthelasma, and to confirm our suspicion, we performed laboratory tests and skin biopsy. Serum lipid levels were normal (total cholesterol, 166 mg/dl; triglyceride, 55 mg/dl; high-density lipoprotein-cholesterol, 59 mg/dl; low-density lipoprotein-cholesterol 96 mg/dl), and other laboratory findings were also within their normal ranges.
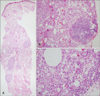
Histologically, a diffuse inflammatory reaction with a Swiss cheese appearance was seen throughout the middle and deep dermis down to the muscle layer (Fig. 2A), which was compatible with paraffinoma rather than xanthelasma. On higher magnification, numerous epithelioid cells, foreign body-type giant cells, and vacuolated histiocytes both scattered individually and in aggregates were seen along with lymphoid follicles (Fig. 2B), and variably sized round to oval vacuoles formed a Swiss cheese pattern (Fig. 2C).
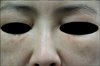
Based on the histological findings, we diagnosed her with paraffinoma and re-evaluated her medical history but did not find any history of contact with oily substances. She was treated with intralesional injection of triamcinolone (2.5 mg/ml); however, not much improvement was seen after 4 weeks. She was then treated twice with 100% trichloroacetic acid (TCA) peel with a 1-month interval, and gradual improvement was seen (Fig. 3).
PLLA injection can stimulate collagen neogenesis that leads to gradual volume restoration; therefore, it is widely used to treat wrinkles of the aging face and facial lipoatrophy in AIDS patients 123. Sculptra® consists of PLLA, sodium carboxymethylcellulose, and non-pyrogenic mannitol, and does not contain an oily component36.
Immediate volume increase after PLLA injection disappears as the suspension fluid is absorbed. PLLA microspheres are degrade by the host's inflammatory response, and the intended foreign body reaction causes the true bulking effect of PLLA126.
At the introduction of PLLA injection and its initial use, the relatively high incidence of papules and nodules after injection was a leading problem1. However, as its use increased, safer injection technique were developed, and the frequency of these kinds of adverse events decreased considerably1. Other frequent side effects were bruising, redness, and edema, and these injection-related complications resolved spontaneously in a short time1234. Recently, some late-onset complications, such as granuloma, have been reported in the literature246. To the best of our knowledge, paraffinoma as a delayed complication of PLLA injection has never been reported.
Usually, granulomatous reaction of PLLA demonstrates a foreign body granuloma, with numerous multinucleated giant cells around translucent particles of different sizes67. The PLLA particles have a fusiform, oval, or spiky shape, which is similar to cholesterol clefts, but shorter and wider67. These translucent particles are very different from variably sized vacuoles that form a Swiss cheese appearance in paraffinoma67.
Although the exact pathomechanism of paraffinoma is still unknown, an inflammatory reactive process associated with exogenous or endogenous lipids in the dermis and subcutaneous layer is believed to be involved8. Paraffinoma typically occurs in areas where lipid material has entered traumatically or has been injected for a specific purpose. The injected exogenous oils include paraffin, mineral oil, silicone, vaseline, vitamin E, and autologous fat9. Paraffinoma occurring after use of paraffin-containing materials, such as antibiotic ointment, has been reported. This type of paraffinoma can be explained by the natural host response to wall off exogenous substances that are too large to be ingested by macrophages7. However, another type of paraffinoma that occurs after exenatide injection, triamcinolone injection, aluminum hydroxide injection, and trauma has been reported; however, this type cannot be explained by the above mechanism8910. Its mechanism involves endogenous fat degeneration8. The breakdown of endogenous lipid following trauma, vascular insufficiency, infection, foreign body injection, and other such events results in the release of fat droplets into the intracellular spaces, and this leads to a macrophage response8. A lipogranulomatous reaction can occur through this process, and our case could be partly explained by this mechanism.
For the treatment of paraffinoma, complete surgical excision is considered to be the most definitive procedure. However, complete removal is not always possible, and patients usually refuse surgical excision because of its invasiveness. Many other treatment options have been reported, such as intralesional and systemic corticosteroids, oral tetracycline, imiquimod cream, liposuction, 10,600-nm carbon dioxide fractional laser, and bipolar radiofrequency device6. Focal treatment using TCA 100% chemical agent can be used to treat many dermatological conditions. Depth of coagulation necrosis is determined by the concentration and number of repetition of TCA11. Repetitive use of higher concentration of TCA can affect up to reticular dermis12, so some authors suggested to choose TCA concentration proportional to the severity of target lesion when treating xanthelasma13. TCA peel is a simple, straightforward, and non-surgical treatment option for xanthelasma, and it could show similar benefits for paraffinoma. In summary, we have described the first case of paraffinoma as a late-onset complication of Sculptra® injection in the English literature. Physicians and dermatologists who practice aesthetic dermatology should be aware of the delayed complications of PLLA injection, including paraffinoma.
Figures and Tables
Fig. 1
A physical examination of the patient shows relatively well-demarcated yellowish plaque lesions in both the lower eyelids, and the lesion on the right side was more prominent.
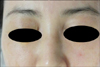
Fig. 2
(A) A hematoxylin and eosin-stained section shows a diffuse inflammatory reaction with a Swiss cheese appearance and 4 reactive lymphoid follicles throughout the middle and deep dermis down to the muscle layer (×40). (B) Numerous epithelioid cells, a foreign body-type giant cell, and vacuolated histiocytes both scattered individually and in aggregates forming a Swiss cheese pattern are seen along with a lymphoid follicle (H&E, ×200). (C) Variably sized vacuoles, which correspond to lipid removed with tissue processing, as well as numerous foamy histiocytes, lymphocytes, and hyalinized collagen fibrils are seen (H&E, ×400).
References
1. Bartus C, William Hanke C, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013; 39:698–705.

2. Alijotas-Reig J, Garcia-Gimenez V, Vilardell-Tarres M. Late-onset immune-mediated adverse effects after poly-L-lactic acid injection in non-HIV patients: clinical findings and long-term follow-up. Dermatology. 2009; 219:303–308.

3. Lowe NJ, Maxwell CA, Lowe P, Shah A, Patnaik R. Injectable poly-l-lactic acid: 3 years of aesthetic experience. Dermatol Surg. 2009; 35:Suppl 1. 344–349.
4. Duracinsky M, Leclercq P, Herrmann S, Christen MO, Dolivo M, Goujard C, et al. Safety of poly-L-lactic acid (New-Fill®) in the treatment of facial lipoatrophy: a large observational study among HIV-positive patients. BMC Infect Dis. 2014; 14:474.

5. Kim JH, Choi JS, Yun JH, Kang HK, Baek JO, Roh JY, et al. Foreign body reaction to injectable hyaluronic acid: late granuloma formation. Ann Dermatol. 2015; 27:224–225.

6. Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009; 123:1842–1863.

7. Requena L, Requena C, Christensen L, Zimmermann US, Kutzner H, Cerroni L. Adverse reactions to injectable soft tissue fillers. J Am Acad Dermatol. 2011; 64:1–34. quiz 35-36.

8. Park KY, Choi SY, Seo SJ, Hong CK. Posttraumatic lipogranuloma on the lower leg. J Dermatol. 2013; 40:141–142.

9. Shan SJ, Guo Y. Exenatide-induced eosinophilic sclerosing lipogranuloma at the injection site. Am J Dermatopathol. 2014; 36:510–512.

10. Abel AD, Carlson JA, Bakri S, Meyer DR. Sclerosing lipogranuloma of the orbit after periocular steroid injection. Ophthalmology. 2003; 110:1841–1845.

11. Dailey RA, Gray JF, Rubin MG, Hildebrand PL, Swanson NA, Wobig JL, et al. Histopathologic changes of the eyelid skin following trichloroacetic acid chemical peel. Ophthal Plast Reconstr Surg. 1998; 14:9–12.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download