Abstract
Background
Recently, the pulse-in-pulse mode of intense pulsed light (IPL) has been used increasingly for the treatment of melasma.
Objective
To observe the morphologic changes in the melanophore in adult zebrafish after irradiation with conventional and pulse-in-pulse IPL and Q-switched Nd:YAG (QSNY) laser.
Methods
Adult zebrafish were irradiated with conventional and pulse-in-pulse mode of IPL. The conditions for conventional IPL were 3 mJ/cm2, 560 nm filter, and pulse widths of 7, 20, and 35 msec. The pulse-in-pulse conditions were 3 mJ/cm2 and on-time 1/off-time 2. The QSNY laser was used with the settings of 1,064 nm, 0.4 J/cm2, a 7 mm spot size, and one shot. Specimens were observed using a light microscope, a transmission electron microscope (TEM), a scanning electron microscope (SEM) and a confocal microscope.
Results
After conventional IPL irradiation with a 7 msec pulse width, melanophore breakage was observed using light microscopy. Under TEM, irradiation with conventional IPL for 7 msec and pulse-in-pulse IPL induced melanophore thermolysis with vacuolization. However, changes in the melanophore were not observed with 35 msec IPL. Under SEM, unlike the control and QSNY groups, IPL-irradiated zebrafish showed finger-like fusion in the protein structure of scales. Specimens examined by a confocal microscope after conventional IPL irradiation showed a larger green-stained area on TUNEL staining than that after pulse-in-pulse mode IPL irradiation.
Intense pulsed light (IPL) refers to polychromatic light that radiates at a wavelength from 400∼1,200 nm depending on the filter in contrast to laser light, which is monochromatic. In the past several decades, IPL has become popular for pigmentation removal and skin rejuvenation. However, the underlying mechanisms are unclear.
More recently, the pulse-in-pulse mode of IPL has been used increasingly for the treatment of melasma and postinflammatory hyperpigmentation. Conventional IPL radiates light every 2∼35 msec, while the pulse-in-pulse mode radiates light over a shorter time span (1∼900 µsec); the latter emits multiple fractionated subpulses within a pulse duration of 10 msec. The pulse-in-pulse mode IPL causes a gradual increase in the skin temperature and might be safer than conventional IPL1. The shorter pulse width of IPL is expected to decrease the energy absorbed by chromophores in the surrounding tissue. However, this phenomenon has not been established. In the present study, we observed the morphologic changes in the melanophore in adult zebrafish after irradiation with conventional and pulse-in-pulse IPL and Q-switched Nd:YAG (QSNY) laser in order to evaluate the efficacy and treatment mechanisms.
The skin of the zebrafish contains neural crest-origin pigment cell system that includes melanocytes which can be potential targets for enlightening the developmental biology and pathology of pigmentation2. Zebrafish is a convenient and cost-effective model suitable for researching pathomechanisms of human skin diseases3.
Adult zebrafish were preserved in glass aquaria containing aerated tap water under standard laboratory conditions at 28℃ with an alternating cycle of 14 h light and 10 h darkness. Zebrafish were fed flake food and live brine shrimp three times a day, 6 days per week. Fish floating upside down and motionless were considered dead and were removed. The zebrafish were anesthetized with 4% 3-amino benzoic acid-ethylester and laid on a glass slide prior to irradiation. The IPL sapphire tip was too large for the body surface; hence, zebrafish were covered with a non-penetrable cover that left a 4 mm diameter area exposed. Irradiation was performed by exposing the 4 mm caudally exposed peduncle of each zebrafish to a pulse of light from an E-toning IPL apparatus (Union Medical, Uijeongbu, Korea) operating in the conventional or pulse-in-pulse modes and using various pulse durations. The conditions for conventional IPL were 3 mJ/cm2, 560 nm filter, and pulse widths of 7, 20, and 35 msec. The pulse-in-pulse conditions were 3 mJ/cm2 and on-time 1/off-time 2. To compare the morphologic changes observed under a scanning electron microscope after IPL irradiation with QSNY laser, 1,064 nm QSNY laser (Lutronic, Goyang, Korea) beam was delivered to the caudal peduncle of zebrafish with the settings of 0.4 J/cm2, a 7 mm spot size, and one shot. During irradiation, the fish were kept moist. They were immediately transferred to acrylic tanks after irradiation.
The study protocol was approved by the Korea University Institutional Animal Care and Use Committee (KUIACUC-20121203-1).
Zebrafish were observed using a model BX50 light microscope (Olympus, Tokyo, Japan) at 1, 3, 5, 7, 14, 21, and 35 days after irradiation.
To investigate the ultrastructural changes after IPL irradiation, biopsy specimens were obtained from zebrafish one day after irradiation. Samples were immediately fixed in 2.5% glutaraldehyde in 0.1 M pH 7.4 phosphate buffer and post-fixed in 1% osmium tetroxide. After dehydration with a graded ethanol series, the tissues were embedded in Epon812 (Electron Microscopy Sciences, Fort Washington, PA, USA). Ultrathin transverse sections stained with uranyl acetate and lead citrate were viewed using a model H-7650 electron microscope (Hitachi, Tokyo, Japan).
To investigate the morphologic changes after IPL and QSNY laser irradiation, biopsy specimens obtained 1 day after irradiation were fixed with glutaraldehyde, dehydrated using a graded ethanol-water mixture series up to 100% ethanol, and critical-point dried. The samples were examined using a model S-4700 field emission scanning electron microscope (Hitachi).
Biopsy specimens obtained 1 day after irradiation were fixed with 4% paraformaldehyde, 1.5% agar, 5% sucrose, and frozen using 2-methyl-butane and liquid nitrogen. A terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) detection kit (In Situ Cell Death Detection kit; Roche, Mannheim, Germany) was used according to the manufacturer's instructions. Confocal microscopy was performed using a model LSM700 device (Zeiss, Jena, Germany).
After conventional IPL irradiation with a 7 msec pulse width, melanophore breakage was observed using light microscopy. Five to seven days after irradiation, no melanophores were observed at the irradiation site (Fig. 1A, B). Melanophore reformation was observed 2 weeks after the treatment. In contrast, irradiation with a 35 msec pulse width did not induce the clearance of melanophores. Instead, individual melanophores displayed blurred but unbroken margins (Fig. 1C, D), with no significant changes apparent even after 5 weeks.
Irradiation with conventional IPL for 7 msec and pulse-in-pulse IPL induced melanophore thermolysis, with vacuolization, central electron lucency and empty spaces evident due to melanophore destruction (Fig. 2B, C). A 35 msec pulse width induced melanophore changes but no vacuolization (Fig. 2D). Serial observation of changes after irradiation with 35 msec pulse revealed some destruction and cell debris. However, the degree of destruction was significantly less than that observed using shorter pulse widths.
Control zebrafish or zebrafish irradiated using a QSNY laser did not show microstructure changes. However, zebrafish irradiated with conventional IPL for 7, 20, and 35 msec showed finger-like fusion in the protein structure of scales regardless of the pulse duration (Fig. 3).
Specimens examined after conventional IPL irradiation showed larger green-stained superficial necrotic materials on TUNEL staining than those after pulse-in-pulse mode IPL irradiation (Fig. 4). They also showed broader degenerative changes and interstitial edema in the muscle layer. This implies that conventional IPL caused more cell death than the pulse-in-pulse mode.
IPL and QSNY laser are widely used non-ablative light sources in treating pigmentation like melasma, because of short downtime and relatively good efficacy4. The mechanism by which IPL improves pigmentation is unclear, but intraepidermal microcrust formation and subsequent shedding have been suggested5. The mechanism of the QSNY laser in pigmentation can be elaborated by the selective photothermolysis theory6. However, IPL emits light with a pulse duration longer than the thermal relaxation time (TRT) of the human melanosome; hence, it cannot be explained by this theory. If the pulse duration is longer than the TRT of the chromophore, the produced heat extends to the surrounding tissue, and it is not confined within the chromophore itself. Indeed, when IPL is applied for tattoo removal, heat transfer to the surrounding dermis and epidermis can result in blistering, dyschromia, and scarring7
One theory for mechanism of IPL posits that the energy generated by IPL is not strong enough to destroy the melanosomes, but it is sufficient to initiate the epidermal turnover for removing melanosomes. The other suggested mechanism is the extended concept of selective photothermolysis, in which neighboring chromophores are affected by heat diffusion from the target chromophore8. Selective photothermolysis requires a wavelength preferably absorbed by the chromophore, pulse duration enough to confine heat within or near the target structures during the laser pulse irradiation, and sufficient fluence to affect the targets6. Although the TRT of the human melanosome is in the nanosecond range, the basal layer TRT is 1∼10 msec. If the target of IPL therapy is the basal layer, the working mechanism can be explained by selective photothermolysis of the basal layer as a whole. However, these do not exactly explain the mechanism of IPL in melanosomes. This study was performed to clarify the mechanism of IPL in melanosomes.
The human melanosome measures approximately 1×0.5×0.75 µm in diameter and its TRT is 10∼100 nsec. Zebrafish melanophores are large enough (0.03∼0.05 mm diameter) to be visible to the naked eye. Considering that the TRT in seconds is nearly identical to the target dimension in square millimeters, the TRT of the zebrafish melanophore is calculated to be 0.9∼2.5 msec. Melanophore photolysis in zebrafish was caused by pulse-in-pulse IPL, which has a µsec pulse duration and an IPL duration up to 20 msec. Pulse duration of 35 msec did not cause cell lysis, echoing the findings with the human melanosome following IPL irradiation5. However, TEM showed changes in zebrafish melanophores consistent with coagulation damage. Human melanosome signaling initiates the epidermal turnover that leads to formation of a microcrust containing melanosomes. IPL radiation can destroy the melanin cap structure, with removal of the desquamated microcrust via epidermal turnover, as was demonstrated using reflectance-mode confocal microscopy5. The authors described the presence of melanophores with cell debris in the microcrust and the supposed appearance of intact melanophores in TEM examination. In the present study, light microscopy confirmed the intact melanophore structure but showed blurred margin after long pulse duration (35 msec) IPL irradiation, with higher resolution examination using TEM demonstrating melanophore damage. The present observations support the suggestion that the applied photothermal energy, which is insufficient to cause lysis of melanosomes, can lead to generation of a signal for the release of the surface crust following epidermal turnover.
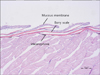
Unlike human skin, the fish have scales which are calcified plates originating in the dermis and covered by the mucous membrane (Fig. 5). A horizontal view of the fish skin reveals melanophores within the dermis below the scales. In contrast to human melanocytes that are in contact with keratinocytes via dendrites to transfer melanosomes, the zebrafish epidermis is blocked by the calcified plate from underlying melanophores. When the IPL pulse duration is shorter than the TRT, photothermolysis occurs and the melanophores are destroyed. However, light with a pulse duration longer than the TRT of zebrafish melanophores allows the melanophores to remain in the space with some ultrastructurally apparent damage but no cell lysis. In human skin, these slight changes may accelerate the epidermal turnover in order to remove the slightly damaged melanosomes. In addition, in the present study, pulse-in-pulse mode or long pulse-IPL (7, 20, and 35 msec) caused finger-like fusion of fish scales that was not observed in the control tissue or following irradiation using a QSNY laser. Thus, IPL may have a strong photothermal effect that causes changes related to heating of the melanophores and surrounding tissue. Also, laser irradiation for a duration longer than the TRT of melanosomes may spread thermal energy beyond the confines of melanosomes. Clinically, tissue rejuvenation after IPL treatment may result from thermal diffusion to the dermis, leading to collagen regeneration.
The most troublesome side effect of IPL treatment for pigmentation removal in Asian women is the post-treatment development of melasma. Many women who do not use sunscreen regularly have subtle melasma as determined using ultraviolet photography9. There is a higher chance of developing prominent melasma-like pigmentation in these patients after treatment with high fluence IPL. To prevent the progression of subtle epidermal melasma to prominent melasma-like hyperpigmentation, the treatment should be as gentle as possible. Since the pulse-in-pulse mode IPL caused less cell damage in this study, it may be a good choice for Asian women in their 40s and 50s who are more prone to developing subtle melasma that is not apparent to the unaided eye.
Physicians are increasingly choosing milder therapy in the form of pulse-in-pulse IPL to reduce pain and crust formation. The approach has been effective in the treatment of melasma110 and postinflammatory hyperpigmentation11. The pulse-in-pulse mode IPL produces multiple micro-second subpulses and intermittent breaks in a single milli-second pulse. It delivers constant high-fluence energy with an on-time and off-time, which prevents a sudden increase in the skin temperature that is high enough to cause breakdown11.
In the present study, confocal microscopy showed a less TUNEL staining response following pulse-in-pulse IPL compared to conventional IPL. This suggests that conventional IPL may have greater thermal-related adverse effects than the pulse-in-pulse mode. Since both conventional and pulse-in-pulse mode IPL induced SEM-apparent morphological changes in fish scale, both approaches can be expected to confer a rejuvenation effect. Cell death was less with use of the pulse-in-pulse mode; thus, it is likely that pulse-in-pulse IPL has fewer side effects. Indeed, pain and discomfort were less prevalent during the pulse-in-pulse mode IPL treatment than during conventional IPL.
Melasma is a complex pigmentary skin disease that is characterized by skin barrier malfunction. Any aggressive treatment activates the melanocytes12. Also, post-inflammatory hyperpigmentation often shows pigment incontinence in the dermis due to inflammation of the basal layer. If any treatment aggravates this inflammation, it will lead to more pigmentation. Although it may be difficult to use conventional IPL in such conditions, pulse-in-pulse IPL can be very useful in treating melasma and post-inflammatory hyperpigmentation without flare ups. It is thought that the pulse-in-pulse mode IPL might decrease activities in melanosomes and exhaust melanocytes the way laser toning does1. Although laser toning with a QSNY laser is a popular treatment for melasma in Asia, punctuate hypomelanosis is a troublesome complication that can develop after laser treatment13. Hypopigmented macules do not appear after repeated pulse-in-pulse IPL treatment1.
In conclusion, zebrafish irradiated with long pulse-IPL showed no morphologic changes using light microscopy, while morphological changes in melanophores were evident with use of TEM. While melanophore changes may lead to pigmentation removal following epidermal turnover in the human skin, epidermal turnover in zebrafish may be impossible due to the presence of scales. Instead, slightly damaged melanophores may remain in the zebrafish skin, instead of being lysed or eliminated. IPL-irradiated zebrafish showed more SEM-apparent structural changes in scales than the control or after irradiation with a QSNY laser. IPL may deliver more thermal energy around the chromophore. Pulse-in-pulse mode IPL caused less damage than conventional IPL.
Figures and Tables
Fig. 1
Light microscopy in zebrafish before (A) and 1 week after (B) intense pulsed light (IPL) irradiation with 560 nm filter and 7 msec, and before (C) and 1 week after (D) IPL irradiation with 560 nm filter and 35 msec. Conventional IPL irradiation with a 7 msec pulse width induced melanophore breakage. However, the clearance of melanophore was not observed after irradiation with a 35 msec pulse width.
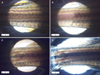
Fig. 2
Representative transmission electron microscope images of (A) control, (B) pulse-in-pulse mode intense pulsed light (IPL), (C) IPL irradiation with 560 nm filter and 7 msec, and (D) IPL irradiation with 560 nm filter and 35 msec. (B, C) Irradiation with pulse-in-pulse IPL and conventional IPL for 7 msec induced melanophore thermolysis (black arrowhead), with vacuolization (white arrowhead), central electron lucency (white arrow), and empty spaces (black arrow) evident due to melanophore destruction. Conventional IPL irradiation with 35 msec pulse width caused some melanophore changes but no vacuolization. Scale bar=100 µm.
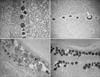
Fig. 3
Representative scanning electron microscope images of (A) control, (B) Q-switched Nd:YAG (QSNY) laser irradiation, and (C) intense pulsed light (IPL) irradiation with 560 nm filter and 20 msec. Control zebrafish or zebrafish irradiated using a QSNY laser did not show microstructural changes. However, IPL-irradiated zebrafish showed finger-like fusion (arrows) in the protein structure of scales.

Fig. 4
Representative confocal microscopic findings of (A) control, (B) pulse-in-pulsed mode intense pulsed light (IPL), and (C) conventional mode IPL irradiation with 560 nm filter and 7 msec. After conventional IPL irradiation, zebrafish showed a larger green-stained area on TUNEL staining than that after pulse-in-pulse mode IPL irradiation (×20).

References
1. Chung JY, Choi M, Lee JH, Cho S, Lee JH. Pulse in pulse intense pulsed light for melasma treatment: a pilot study. Dermatol Surg. 2014; 40:162–168.

2. Ni-Komatsu L, Orlow SJ. Identification of novel pigmentation modulators by chemical genetic screening. J Invest Dermatol. 2007; 127:1585–1592.

3. Li Q, Uitto J. Zebrafish as a model system to study skin biology and pathology. J Invest Dermatol. 2014; 134:e21.

4. Na SY, Cho S, Lee JH. Intense pulsed light and low-fluence Q-switched Nd:YAG laser treatment in Melasma patients. Ann Dermatol. 2012; 24:267–273.

5. Yamashita T, Negishi K, Hariya T, Kunizawa N, Ikuta K, Yanai M, et al. Intense pulsed light therapy for superficial pigmented lesions evaluated by reflectance-mode confocal microscopy and optical coherence tomography. J Invest Dermatol. 2006; 126:2281–2286.

6. Anderson RR, Margolis RJ, Watenabe S, Flotte T, Hruza GJ, Dover JS. Selective photothermolysis of cutaneous pigmentation by Q-switched Nd: YAG laser pulses at 1064, 532, and 355 nm. J Invest Dermatol. 1989; 93:28–32.

7. Wenzel S, Landthaler M, Baumler W. Recurring mistakes in tattoo removal. A case series. Dermatology. 2009; 218:164–167.
8. Altshuler GB, Anderson RR, Manstein D, Zenzie HH, Smirnov MZ. Extended theory of selective photothermolysis. Lasers Surg Med. 2001; 29:416–432.

9. Negishi K, Kushikata N, Tezuka Y, Takeuchi K, Miyamoto E, Wakamatsu S. Study of the incidence and nature of "very subtle epidermal melasma" in relation to intense pulsed light treatment. Dermatol Surg. 2004; 30:881–886. discussion 886.

10. Yun WJ, Moon HR, Lee MW, Choi JH, Chang SE. Combination treatment of low-fluence 1,064-nm Q-switched Nd: YAG laser with novel intense pulse light in Korean melasma patients: a prospective, randomized, controlled trial. Dermatol Surg. 2014; 40:842–850.
11. Park JH, Kim JI, Kim WS. Treatment of persistent facial postinflammatory hyperpigmentation with novel pulse-inpulse mode intense pulsed light. Dermatol Surg. 2016; 42:218–224.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download